
Post-translational modifications (PTM) are key aspects of protein diversity and play an important role in controlling cellular processes, involving a large number of biological functions. Qualitative and quantitative analysis of PTMs contributes not only to the study of disease mechanisms, but also to the discovery and study of biomarkers and the characterization of protein biopharmaceuticals. Due to its sensitivity and wide applicability, mass spectrometry (MS), especially in combination with separation techniques such as chromatography, has become a core technique for analyzing protein and their PTMs. Based on effective protein/peptide enrichment strategies, advanced MS instrumentation, and proteomics technologies, Creative Proteomics can help our global customers to perform fast and reliable deep protein PTM profiling.
MS-based methods are currently the gold standard and most informative technique for the analysis of proteins and their PTM. MS has several advantages in characterizing PTMs, while no other technique can provide, including:
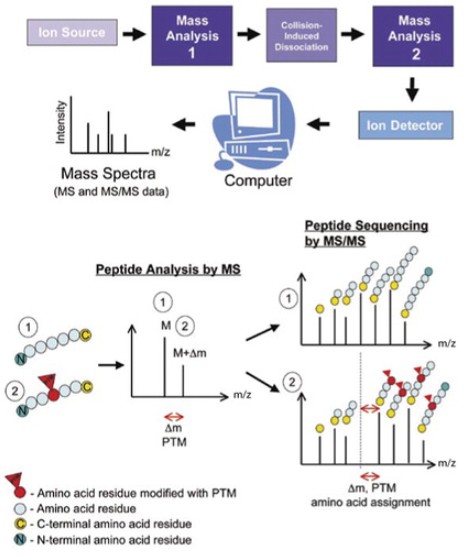 Fig. 1 Tandem mass spectrometry (MS/MS) for mapping posttranslational modifications (PTMs). (Larsen, Martin R., et al., 2006)
Fig. 1 Tandem mass spectrometry (MS/MS) for mapping posttranslational modifications (PTMs). (Larsen, Martin R., et al., 2006)
The disulfide bond is a special post-translational modification (PTM), a form of intra- or inter-chain cysteine linkage of proteins, which plays an important role in the correct folding of the higher structure of proteins and their maintenance of biological activity. As a critical quality attribute of proteins, disulfide bonds must be monitored closely during the development of biotherapeutics. Our protein disulfide bond analysis service is based on high-precision MS. Our service generally includes sample preparation, LC-MS/MS analysis, and rapid disulfide bond analysis, providing a full range of analytical services.
The development of PTM co-occurrence identification strategies has revealed a wealth of information about the interactions between PTMs. There is growing evidence that PTM crosstalk is involved in the development and progression of various diseases. To help our clients gain insight into the complexity of PTM crosstalk and understand how the interactions between PTMs relate to physiological or pathological states, we provide protein PTM crosstalk analysis services.
As protein PTMs have been studied in depth, it has been found that backbone PTMs can control the structure and function of proteins and achieve these functions through unique mechanisms. To help our customers systematically explore the role of backbone PTMs in biology, we offer MS-based analysis methods combined with specific enrichment strategies. In addition, our customers are working on other technologies to enrich and identify more backbone PTMs.
The modifications of protein termini have been shown to be associated with different normal and disease-related processes and constitute a rapidly emerging field of biological regulation. We provide N-terminal PTM analysis service. Our service conducts systematic studies of protein N-terminal PTMs, which can contribute to an in-depth understanding of the multifunctional roles played by proteins in a variety of biological processes.
Creative Proteomics strives to be a guide and assistant in protein PTM research for our customers worldwide. Based on the needs of our customers, we provide the best analytical strategies and solutions to facilitate biomarker discovery, disease research, protein biopharmaceutical characterization, and more. For more information on our PTM proteomics analysis services, please use our contact form. Our customer service representatives are available 24 hours a day, from Monday to Sunday.
Reference
Our products and services are for research use only.