
- Home
- PTMs Proteomics
- Proteomics Analysis of Polyglycylation
Polyglycylation, a post-translational modification, has emerged as a focal point in biological research, offering a nuanced understanding of cellular dynamics. This modification involves the addition of multiple glycine moieties to a target protein, playing a pivotal role in various cellular processes. Polyglycylation contributes significantly to the structural integrity of cellular components, particularly microtubules. This modification influences the stability and function of microtubules, thereby impacting crucial cellular processes such as cell division, intracellular transport, and maintenance of cell shape. Besides, in the realm of ciliary biology, polyglycylation has emerged as a critical regulator. It plays a pivotal role in modulating the structure and function of cilia, influencing cellular signaling and sensory perception. Understanding the implications of polyglycylation in ciliary dynamics opens avenues for targeted therapeutic interventions in ciliopathies.
Standardization of Protocols. Ensuring consistency and reproducibility in polyglycylation analysis requires the standardization of experimental protocols. From sample preparation to data analysis, adhering to standardized procedures enhances the reliability of findings.
Cross-Validation of Techniques. Combining multiple analytical techniques, such as mass spectrometry, western blotting, and immunoprecipitation, enhances the robustness of polyglycylation analysis. Cross-validating results obtained from different methods strengthens the overall reliability of the findings.
Integration with Systems Biology Approaches. Incorporating polyglycylation data into systems biology frameworks provides a holistic understanding of its impact on cellular networks. Integration with omics data allows researchers to unravel the intricate interplay between polyglycylation and other cellular processes.

Polyglycylation as a dynamic post-translational modification, unfolds new dimensions in cellular biology and disease mechanisms. Creative Proteomics provides our clients with accurate and reliable polyglycylation analysis by integrating advanced analytical methods, and the polyglycylation analysis we provide is at the forefront of the research field. A deep and nuanced understanding of the role of polyglycylation in cellular dynamics can help facilitate therapeutic interventions for a wide range of pathological conditions and hold promise for diagnostic advances.
Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation
Journal: Cell
Published: 2009
Background
Polyglycylation is a posttranslational modification that involves the addition of glycine side chains to specific glutamate residues on target proteins. Discovered initially on tubulin, it occurs in the carboxy-terminal tail domains, similar to polyglutamylation. While polyglutamylation is observed in various microtubules, polyglycylation is notably prominent in cilia and flagella, particularly in cells possessing these organelles.
Functional experiments on tubulin glycylation faced challenges due to unknown modifying enzymes. However, modification-specific antibodies hinted at potential functions, such as the regulation of ciliary dynein. In Tetrahymena, eliminating major glycylation sites on β-tubulin resulted in lethality or severe axonemal defects, emphasizing the role of tubulin glycylation in the assembly and functions of axonemal microtubules.
This article introduces a group of enzymes catalyzing tubulin glycylation, demonstrating that glycylases belong to the tubulin tyrosine ligase-like (TTLL) family. TTLL3 and TTLL8 in mice are identified as initiating glycylases with distinct substrate specificities, while TTLL10 is recognized as the elongating polyglycylase. Cooperation between these enzymes generates polyglycine side chains in mammals, while bifunctional enzymes in Drosophila catalyze both initiation and elongation reactions.
The absence of long glycine side chains on human sperm tubulin is attributed to inactivating amino acid substitutions in human TTLL10. Furthermore, RNA interference experiments in Drosophila reveal that depletion of one of the two glycylases leads to reduced polyglycylation, causing defects in sperm maturation and resulting in male sterility. The study suggests that polyglycylation plays a crucial role in the development of Drosophila, with potential broad-ranging functions indicated by the identification of additional substrates of glycylation.
Results
Phylogenetic analysis suggested that TTLL12, while present in species lacking glycylation, showed no glycylation activity. On the other hand, TTLL3, 8, and 10 were associated with known glycylation, with TTLL10 previously identified as a glycylase for NAPs. To assess tubulin glycylase activity, the researchers expressed the cDNAs of TTLL3, 8, and 10 in HEK293 cells and tested the extracts in an in vitro microtubule (MT) glycylation assay. The substrates used, brain tubulin MTs (with low glycylation) and highly glycylated ciliary axonemal MTs of Tetrahymena thermophila, helped distinguish initiation and elongation activities. TTLL3 and 8 demonstrated initiation activity, promoting glycine incorporation into brain MTs but showing low activity on axonemal MTs. In contrast, TTLL10 exhibited efficient elongation activity, with significant glycine incorporation into axonemal MTs but almost no activity on brain MTs. These findings suggest that TTLL3 and 8 are initiating glycylases, while TTLL10 functions as an elongating glycylase (polyglycylase) for tubulin, paralleling functional specialization observed among polyglutamylases (Figure 1).
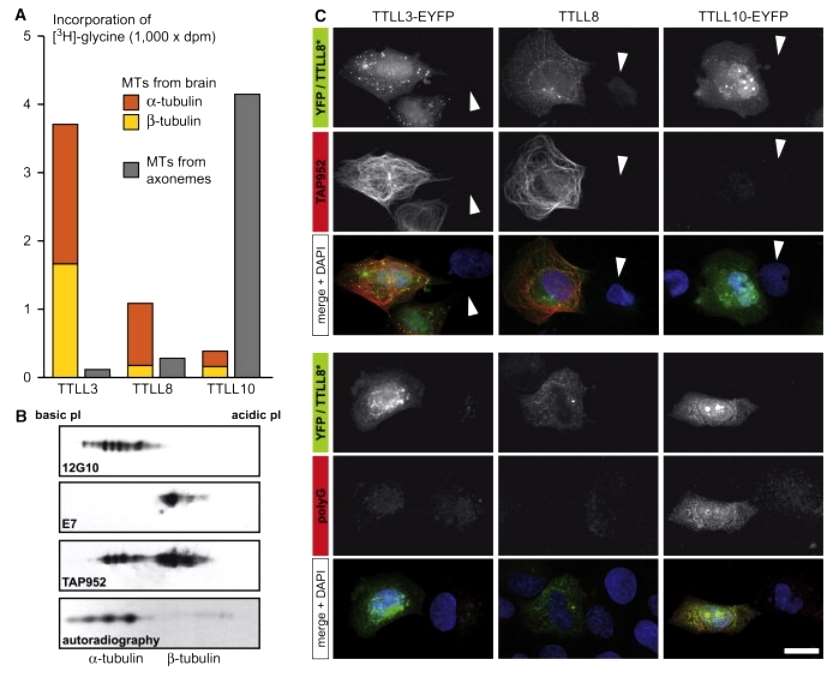 Figure 1
Figure 1
To test whether the two types of mammalian glycylases can function cooperatively to create long side chains on MTs, we co-overexpressed either TTLL3 or 8 together with TTLL10 in U2OS cells. In both cases, we observed a strong labeling of MTs with the polyG antibody (Figure 2). These results indicate that MTs have acquired polyglycine side chains, which was not the case when TTLL3, 8, or TTLL10 were expressed alone.
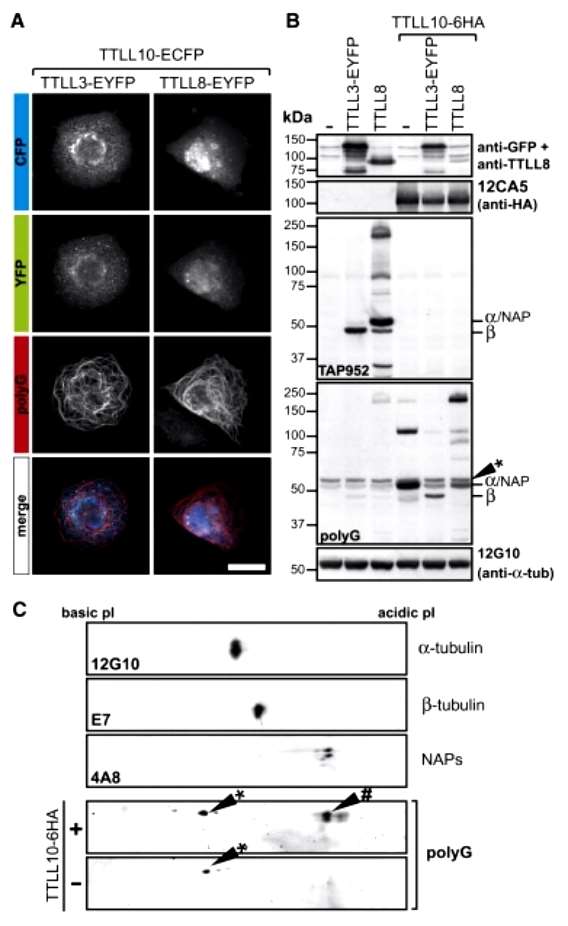 Figure 2
Figure 2
The author confirmed this by comparing the status of glycylation in protein extracts prepared from mouse, rhesus monkeys, and human sperm using the TAP952 and the polyG antibody. Several mouse and rhesus protein bands were strongly labeled with polyG, while only weak signals were detected with TAP952. In contrast, human sperm proteins were not labeled with polyG but strongly with TAP952. No differences between the three species were found for the related tubulin modification, polyglutamylation (Figure 3).
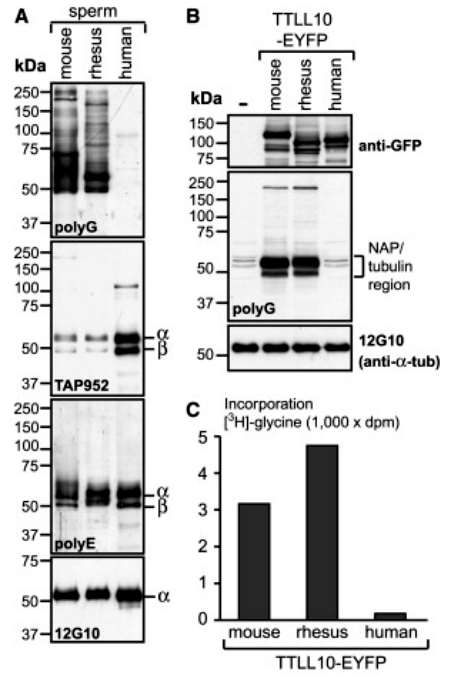 Figure 3
Figure 3
In mammals, TTLL10 is identified as the sole enzyme capable of elongating glycine side chains. However, in Drosophila, an organism with polyglycylated microtubules (MTs), only two orthologs of the TTLL3/8 group are present, and no TTLL10 gene has been found. To understand the mechanism behind glycine side chain elongation in the absence of TTLL10, the study investigated the activity of Drosophila melanogaster enzymes, dmTTLL3A (CG11323) and dmTTLL3B (CG11201), in U2OS cells. Both enzymes induced monoglycylation on MTs, with dmTTLL3B showing restricted nuclear localization and labeling only the nucleus. Unlike mammalian orthologs, dmTTLL3A generated polyglycylated MTs, while dmTTLL3B polyglycylated nuclear proteins but not MTs. Immunoblots revealed that dmTTLL3A initiates and elongates glycine side chains on α- and β-tubulin, while dmTTLL3B initiates glycylation on various proteins, including α-tubulin, but functions as an elongating glycylase only for non-tubulin substrates. This polyglycylation mechanism in Drosophila involves bifunctional initiating/elongating glycylases (Figure 4).
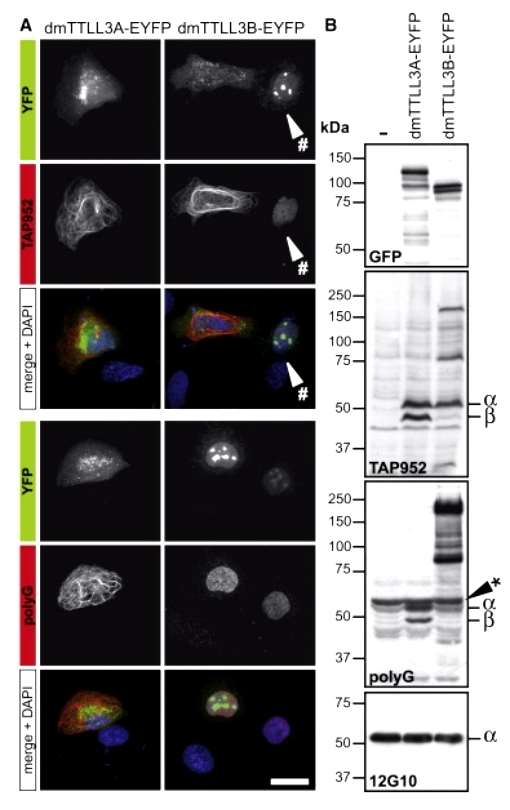 Figure 4
Figure 4
Polyglycylation is notably abundant in testes, particularly associated with the axonemes of sperm flagella. In Drosophila, one of the polyglycylase genes, dmTTLL3B, exhibits high expression in testes, indicating that it is a major polyglycylase in this organ. To investigate the functional significance of dmTTLL3B, the study attempted to deplete it using RNA interference (RNAi) in Drosophila. A transgenic fly carrying inverted repeats corresponding to dmTTLL3B under the control of UAS sequences inducible by Gal4 (UAS-IR-dmTTLL3B transgene) was used. Strikingly, 50% of embryos carrying UAS-IR-dmTTLL3B and the ubiquitous da-Gal4 driver did not survive to adulthood, suggesting an essential role for dmTTLL3B in development (Figure 5).
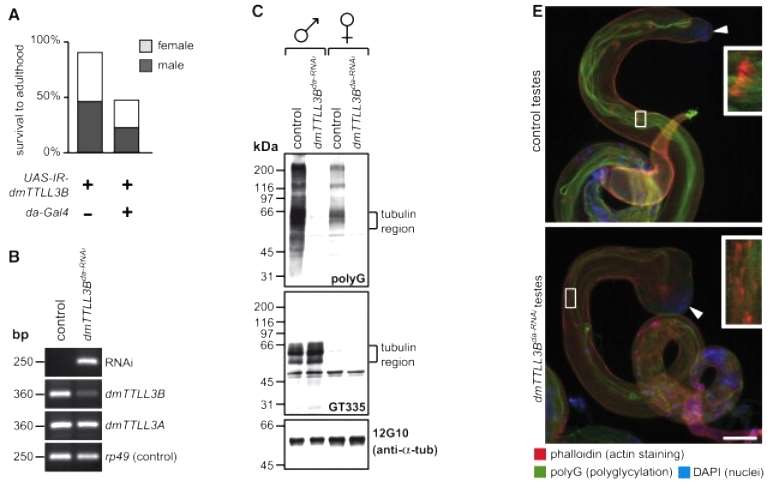 Figure 5
Figure 5
Conclusion
The research identifies an evolutionarily conserved family of glycine ligases responsible for modifying tubulin through various enzymatic mechanisms. In mammals, two distinct types of enzymes facilitate the initiation and elongation steps of polyglycylation, while Drosophila glycylases serve both functions in a bifunctional manner. The study also reveals that the human elongating glycylase has lost enzymatic activity due to two amino acid changes, suggesting that monoglycylation might sufficiently fulfill the functions of protein glycylation.
Functional experiments in Drosophila, where a glycylase is depleted using RNA interference, result in adult flies with significantly reduced total glycylation levels, male sterility, and defects in sperm individualization and axonemal maintenance. Severe RNAi depletion is lethal at early developmental stages, indicating the essential nature of protein glycylation. The study, coupled with the observation of multiple glycylated proteins, suggests a general role of glycylation in protein functions.
Reference
Our products and services are for research use only.