
- Home
- PTMs Proteomics
- Histone PTM Analysis
- Histone PTM Data Analysis
Histone post-translational modifications (PTMs) have become one of the hot spots in epigenetic research because of their ability to regulate gene transcription and expression by regulating the structure of chromatin. The abnormal patterns of histone PTMs can lead to defects in these cellular processes, which may result in human disorders, including Alzheimer's disease, autoimmune diseases, and various forms of cancer. Mass spectrometry (MS)-based proteomics workflow has been established to investigate histone PTMs. As laboratory techniques and MS technologies advance, deeper sequencing coverage of protein and histone PTMs can be obtained, generating large amounts of data that need to be analyzed and visualized. Based on sophisticated bioinformatics tools and experienced experts, Creative Proteomics offers a histone PTM data analysis service dedicated to helping researchers and professionals process and analyze histone PTM data efficiently and accurately.
To analyze histone PTMs, a variety of MS-based techniques have been applied, including bottom-up, middle-down, and top-down approaches.
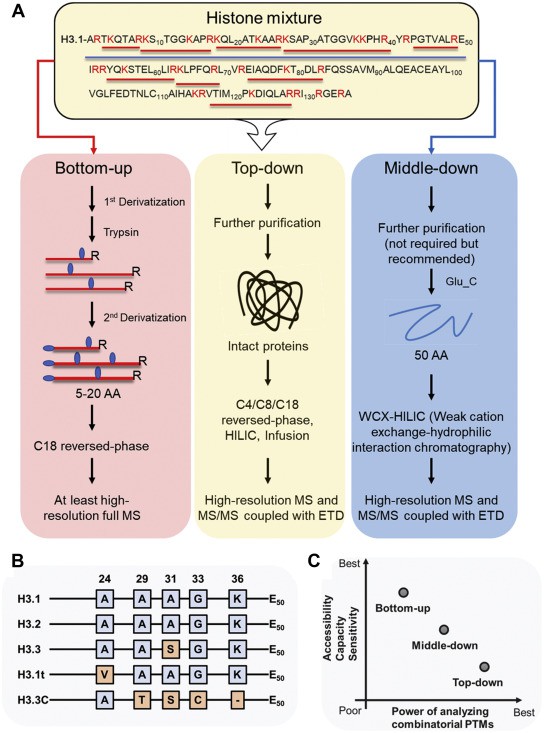 Fig. 1 MS-based proteomics analysis of histone PTMs. (Lu, Congcong, et al.)
Fig. 1 MS-based proteomics analysis of histone PTMs. (Lu, Congcong, et al.)
We have years of experience in large-scale proteomics research and histone PTM data analysis. Our bioinformatics analysis team keeps up with research advances in bioinformatics analysis methods and has established the industry-leading PTM proteomics analysis platform. Our histone PTM data analysis service can not only help our customers to analyze total protein levels but also allows histone PTM data analysis. According to the specific projects of our customers, we implement customized data analysis and visualization, providing results as data tables, stack bar charts, interactive volcano, heatmaps, and so on.
Creative Proteomics is dedicated to the comprehensive and reliable identification and quantification of histone PTMs. Our PTM proteomics analysis platform has a team of experts and state-of-the-art equipment to help you navigate successful research projects through strong workflow, powerful analysis, and tailored services. Need help analyzing your data? We offer affordable biostatistics & bioinformatics services. Contact us right now!
References
Our products and services are for research use only.