
- Home
- PTMs Proteomics
- Proteomics Analysis of S-Glutathionylation
S-glutathionylation is a reversible post-translational modification involving the addition of glutathione (GSH) to cysteine residues of proteins and plays a pivotal role in cellular redox regulation. This process is integral to maintaining cellular homeostasis and has garnered significant attention in the field of molecular biology. Understanding the intricacies of S-glutathionylation is crucial for unraveling its implications for health and disease.
An essential tool for studying redox biology is S-glutathionylation analysis. Exploiting cutting-edge analytical techniques and using this information in a variety of domains, such as medication creation and illness research, creates new opportunities for scientific investigation. As we continue to delve deeper into the complexities of S-Glutathionylation, the potential for therapeutic interventions and a deeper understanding of cellular redox regulation becomes increasingly apparent.
Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages
Journal: Nature Communications
Published: 2021
Background
Under conditions of hyperoxia and LPS-induced acute lung injury, protein S-glutathionylation modification is increased and the expression of the corresponding deglutathionase Grx1 is decreased, especially FABP5 is susceptible to glutathione modification at the cysteine 127 site under oxidative conditions thereby promoting its fatty acid binding and nuclear localization, and facilitating the interaction of FABP5 with PPARβ/δ and activation of PPARβ/δ downstream target genes to inhibit LPS-induced macrophage inflammation. In this paper, we will specifically elucidate the mechanism of FABP5 cysteine glutathione modification on the regulation of the macrophage inflammatory process during the case process of acute lung injury.
Results
C57 mice were randomly grouped and exposed to normoxic or hyperoxic conditions, respectively, and the ratio of reduced glutathione GSH/oxidized glutathione was decreased in hyperoxia, and high expression of GSH synthase (Gss) was found. The results suggest an association between redox regulation and hyperoxic acute kidney injury. Analysis of key enzymes involved in glutathionylation revealed a significant decrease in the expression level of the deglutathione enzyme Grx1, while the expression levels of Grx2 and the glutathione-S-transferase Gsto 1-1 remained unchanged. And the enzyme activity of Grx1 was reduced under hyperoxia exposure conditions. These results suggest that Grx1 is involved in the pathologic process of lung injury induced by hyperoxia, and the lung injury condition of Grx1 knockout mice was significantly improved under hyperoxia exposure. Moreover, granulocytes, and cytokines were significantly reduced under this condition. And the overall protein S-glutathionylation level was increased. In Grx1 knockout mice, protein S-glutathionylation levels were solidly elevated after hyperoxia exposure compared to controls. Thus, these results suggest the importance of Grx1 and S-glutathionylation in hyperoxia-induced lung injury. We suggest that the reduction in Grx1 expression levels and activity in mouse lung tissue plays a protective role against oxidative stress (Figure 1).
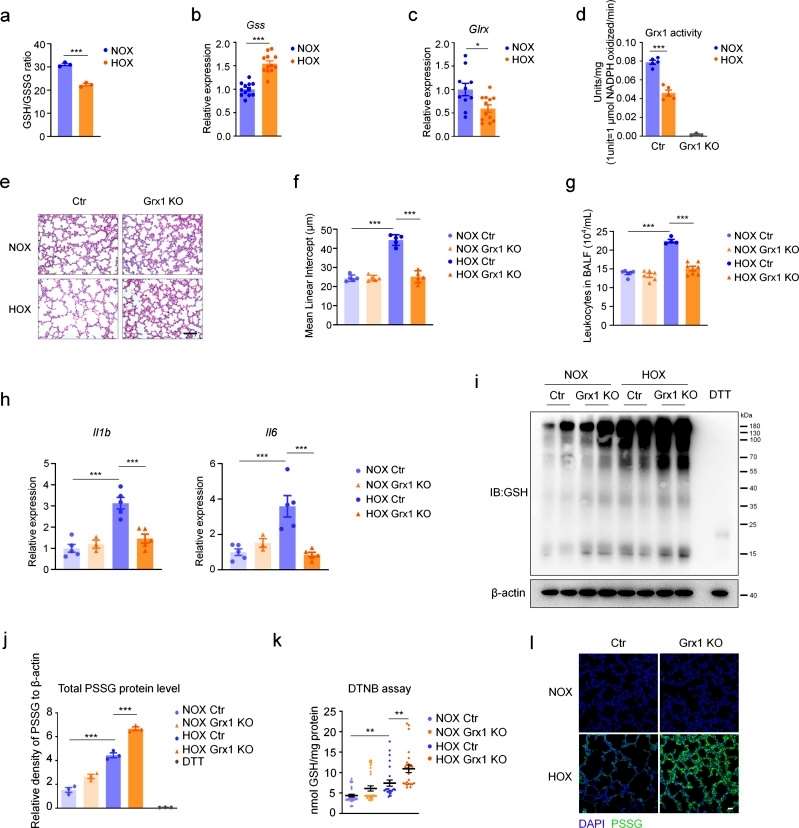 Figure 1
Figure 1
The authors next explored which cell type is most sensitive to oxidative stress during the pathologic process of acute lung injury by detecting intracellular ROS levels with a fluorescent probe, and found that total ROS levels were significantly increased and the intracellular GSH/GSSG ratio was significantly decreased in macrophages in the hyperoxia-exposed group compared with the control group, and that Grx1 levels were significantly decreased in hyperoxia-exposed alveolar macrophages (AMs). Moreover, Grx1 deprivation significantly increased protein S-glutathionylation levels in AMs when exposed to hyperoxic conditions. It was next verified whether Grx1 plays an important role in acute lung injury by regulating S-glutathionylation levels in macrophages. Conditional macrophage-specific knockout Grx1 and wild-type mice showed a significant increase in the level of ROS in macrophages under LPS stimulation conditions, and histologic analysis revealed that the inflammatory condition was significantly suppressed in Grx1 knockout mice. And this protective effect was further evidenced by the reduction of granulocytes in alveolar lavage fluid. The expression levels of pro-inflammatory cytokines were also significantly reduced, and the results overall suggest that Grx1-regulated S-glutathionylation in macrophages plays a protective role in acute lung injury (Figure 2).
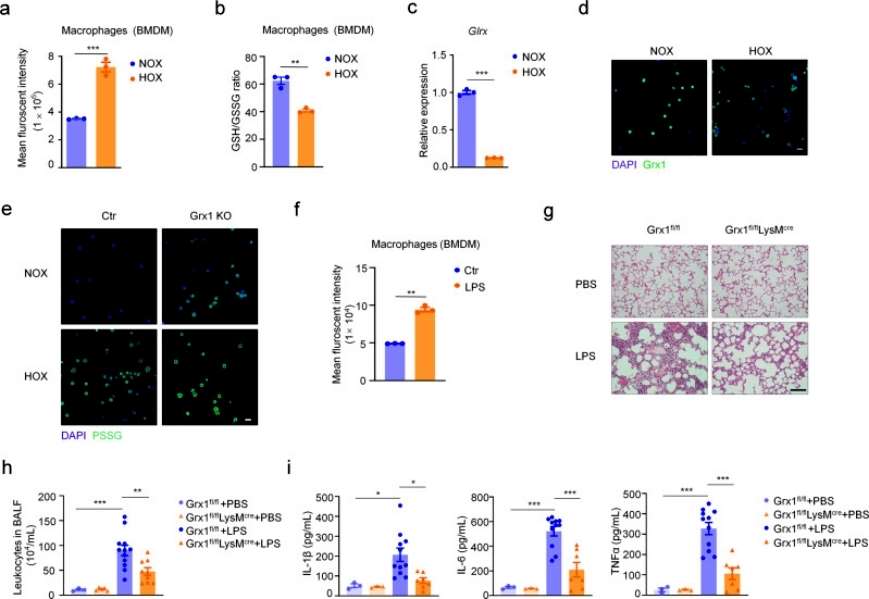 Figure 2
Figure 2
To identify Grx1-specific molecular targets and pathways susceptible to redox-dependent regulation in acute lung injury pathology. S-glutathionylation site-specific analysis was performed within the macrophage proteome using a quantitative redox proteomics approach. glutathionylated proteins were enriched and quantified after BMDM exposure to 200 μM H2O2 treatment for 15 min, and GO analysis of S-glutathionylated proteins revealed that the main enriched distributions were found in the major cellular, metabolic, and single-species processes. Among the top five modified proteins of metabolic processes, FABP5 was expressed at significantly higher levels in macrophages than other proteins. Overall, S-glutathionylation of FABP5 is involved in the regulation of macrophage acute lung injury pathological processes (Figure 3).
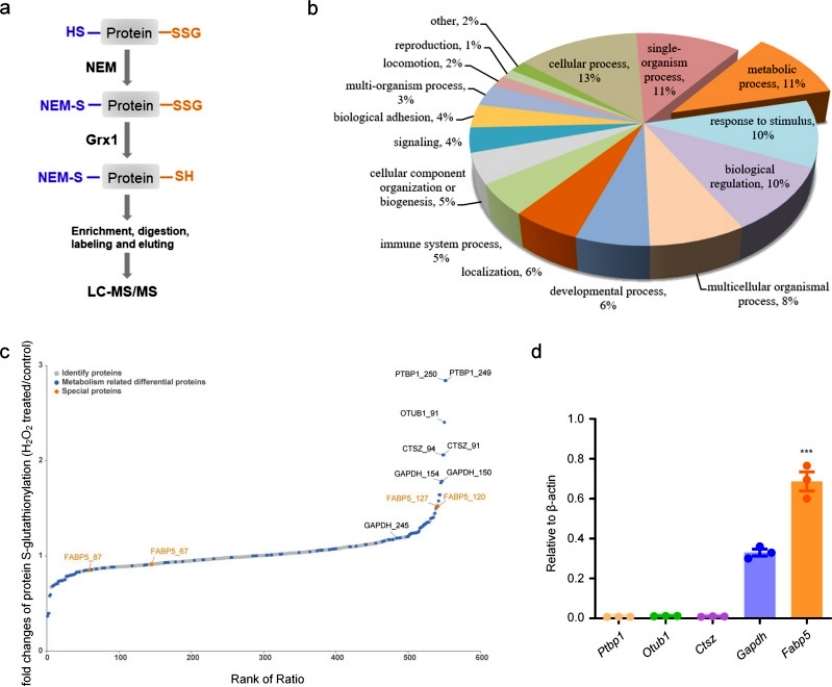 Figure 3
Figure 3
Conclusion
The authors showed that ROS promoted S-glutathionylation of FABP5 in macrophages under oxidative conditions. S-glutathionylation enhanced fatty acid binding and nuclear accumulation of FABP5, activated PPARβ / δ, and inhibited LPS-induced macrophage inflammation. These data confirm Grx1 as a target for anti-inflammatory drug development and suggest that S-glutathionylation of FABP5 acts as an anti-inflammatory mediator in macrophages during acute lung injury. The findings reveal a mechanism by which macrophages prevent self-hyperactivation in response to oxidative stress.
Reference
Our products and services are for research use only.