
- Home
- PTMs Proteomics
- Proteomics Analysis of Glypiation
Glypiation is a frequent post-translational modification that localizes proteins to cell membranes. It is the insertion of a glycosylphosphatidylinositol (GPI) anchor by covalent bonding. Many eukaryotes and certain Archaea have surface glycoproteins with this unique type of glycosylation. Glypiation, the enzymatic process responsible for the addition of GPI anchors to proteins, is a complex and highly regulated mechanism.
Function of Glypiation in Structure. Glycosylphosphatidylinositol (GPI) anchors are essential structural components found in eukaryotic cells. They are introduced through glypiation. These anchors are essential for attaching proteins to the outer layer of the cell membrane, which affects cellular architecture and preserves membrane integrity.
Signaling in Cells via Glypiation. Glypiation has a crucial role in cellular signaling in addition to its structural relevance. Signal transduction across the cell membrane is facilitated by GPI-anchored proteins acting as receptors. The significance of glypiated proteins in cellular response mechanisms and communication is highlighted by their incorporation into signaling networks.
Glypiation plays a more dynamic function in critical cellular activities, such as signaling cascades that control cellular responses and behavior, than it does in providing structural support alone.
Glypiation emerges as a key player in cellular architecture, signaling, and disease pathogenesis. Creative Proteomics integrates advanced analytical methods that can help researchers deepen their overall understanding of the application of glypation and advance the field. As we continue to unravel the complexities of glypiation, its implications in health and disease open new avenues for targeted therapeutic interventions and innovative diagnostic approaches.
Semisynthesis of Functional Glycosylphosphatidylinositol-Anchored Proteins
Journal: Angew Chem Int Ed Engl
Published: 2020
Background
Glypiation is a common posttranslational modification of eukaryotic proteins involving the attachment of a glycosylphosphatidylinositol (GPI) glycolipid. GPIs have a conserved phosphoglycan that undergoes cell- and tissue-specific modifications. Despite the potential roles in biological processes, studying glypiation has been challenging due to difficulties in obtaining homogeneous material. The article introduces a one-pot intein-mediated ligation (OPL) strategy for obtaining GPI-anchored proteins. This method allows glypiation of both folded and denatured proteins with a natural linkage to the glycolipid. The study applied this strategy to glypify eGFP, Thy1, and the Plasmodium berghei protein MSP119. The glypiation process did not alter the structure of eGFP and MSP119 proteins in solution, but it triggered a significant pro-inflammatory response in vitro. The OPL strategy provides a means to produce glypiated proteins, facilitating research into the activity of this modification and potentially serving as vaccine candidates against parasitic infections.
Results
The article describes the process of obtaining GPI-anchored proteins (GPI-APs) through a one-pot intein-mediated ligation (OPL) using Npu N/Npu C split intein fragments. Three fragments were involved in the process: a fusion protein of the protein of interest (POI) and the Npu N fragment (POI‐Npu N), a synthetic Npu C fragment elongated with four amino acids at the C‐extein cleavage site, and a synthetic GPI glycolipid with a cysteine linked to the phosphoethanolamine unit (PEtN) on the GPI Man III unit. The OPL for C-terminal protein modification consisted of three steps. The first step involved the self-assembly of split-intein fragments to form an active intein domain. In the second step, an N-to-S acyl shift produced the thioester POI‐S‐Npu N intermediate. In the third step, this intermediate reacted with a thiol through two pathways, resulting in the desired ligation product. The OPL is considered an expressed-protein ligation reaction, or native chemical ligation, involving in situ formation of protein thioesters mediated by an active split intein domain. To optimize the conditions for C-terminal modification, the study investigated the OPL between eGFP‐Npu N and biotin-4 using different thiol reagents. SDS-PAGE and western-blot analysis revealed the successful formation of the eGFP-biotin product. A kinetic study showed a rapid association of intein fragments, formation of intermediates, and appearance of the ligation product. High protein conversion and yields required extended incubation (Figure 1).
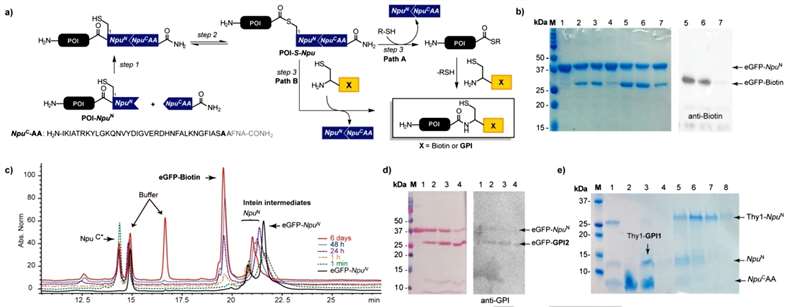 Figure 1
Figure 1
The authors investigates the impact of glypiation on the structure and biological activity of these proteins. The ligations were conducted under optimized conditions, and the resulting products were isolated and analyzed using various techniques such as SDS-PAGE, MALDI MS, LC–MS, and circular dichroism (CD). The CD analysis revealed that OPL and glypiation did not affect the structure of eGFP and MSP119 in solution, a crucial factor for the immunogenicity of these proteins. The study also explores the proinflammatory response induced by isolated GPIs and GPI-anchored proteins, known for triggering cytokine production by macrophages. MSP119‐biotin and MSP119‐GPI3 were tested in vitro, and the results indicated a concentration-dependent cytokine production, with higher levels observed for the glycated protein MSP119‐GPI3. This proinflammatory activity is considered important for protection against malaria but may also contribute to malaria-associated pathology (Figure 2).
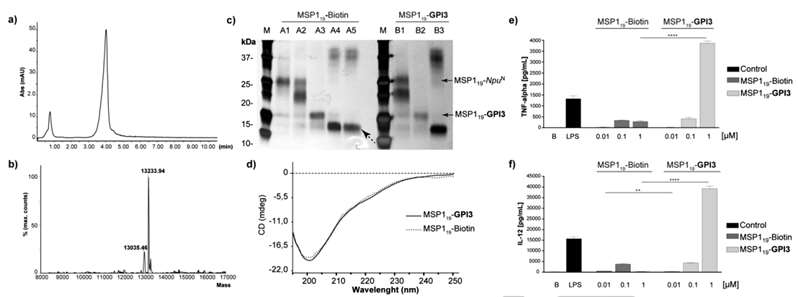 Figure 2
Figure 2
Conclusion
The article emphasizes the significance of the OPL method in overcoming difficulties in accessing GPI-anchored proteins, enabling investigations into the biological role of glypiation and its effects on protein structure and activity. The method provides a means to evaluate the influence of GPI structure on protein folding, the behavior of GPI-APs in membranes, and the activity of glypiated proteins both in vitro and in vivo. Future studies will focus on assessing the effect of glypiation on MSP119 activity in vivo and its potential as a malaria vaccine candidate.
Reference
Our products and services are for research use only.