
- Home
- PTMs Proteomics
- Lipidation
- Proteomics Analysis of Palmitoylation
Palmitoylation is a fatty acylation of cysteine residues undergoing reversible lipid modifications that can modulate target proteins in a variety of ways, such as regulating protein transport, stability, and cellular localization, involving many biological processes that are relevant to the development of many diseases. The identification and analysis of palmitoylated modified proteomes, including modification sites, is of great importance. To meet the need for palmitoylation protein research and identification of palmitoylation sites in multiple samples, Creative Proteomics offers a one-stop, customized protein palmitoylation analysis service.
Protein palmitoylation (S-acylation) is a thioester linkage of a lipid to a protein cysteine residue, of which 16-carbon fatty acid palmitate is the most common type. As one of the only known reversible lipid modifications of proteins, palmitoylation is unique among other lipid modifications, acting as a regulatory PTM. By increasing hydrophobicity at specific points in the protein structure and thus allowing protein binding/interaction with the membrane, such protein modifications can regulate protein cell surface expression, transport, and compartmentalization. In addition to soluble proteins associated with membranes via palmitoylation (e.g. Ras), integral membrane proteins are also widely modified by palmitoylation, including modulating the topology/structure of integral membrane proteins as well as regulating the accessibility of protein domains to enzymes that mediate other regulatory PTMs.
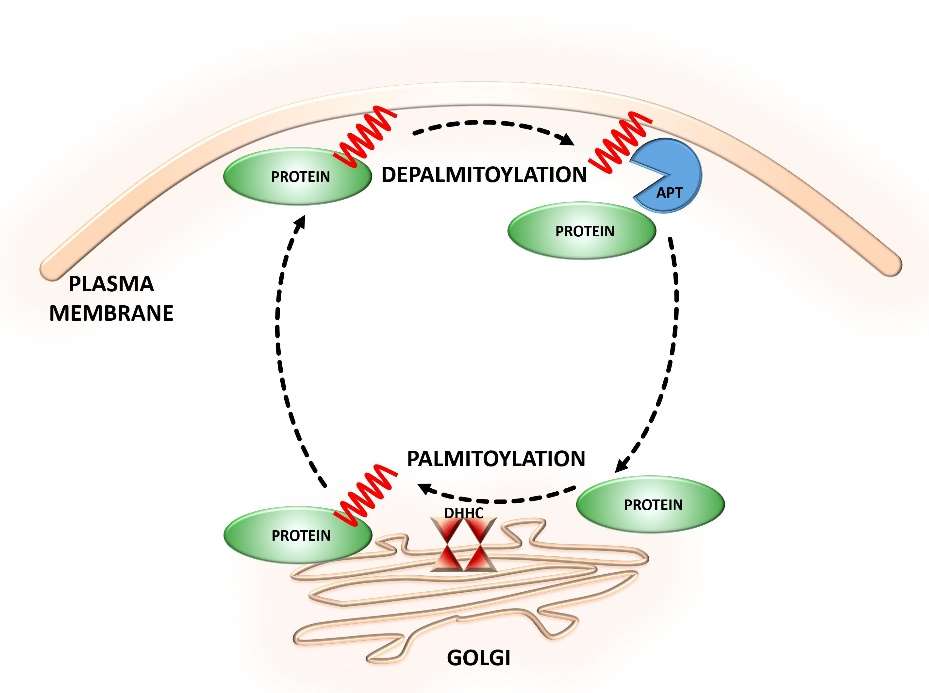 Fig. 1 Dynamic S-palmitoylation of proteins.
(Zaręba-Kozioł, Monika, et al., 2018)
Fig. 1 Dynamic S-palmitoylation of proteins.
(Zaręba-Kozioł, Monika, et al., 2018)
A variety of different methods have been developed to investigate protein palmitoylation. Creative Proteomics provides fast and efficient protein palmitoylation identification and analysis services with a professional and high-throughput liquid chromatography-tandem mass spectrometry (LC-MS/MS) platform. To perform a more accurate palmitoylation analysis of proteins, we used a combination of labeling methods and then compared palmitoylated proteins identified from each method with each other by MS. Our integration and optimization services enable the enrichment and reliable identification of many palmitoylated protein and palmitoylation sites. Specifically, our services include protein extraction and trypsin digestion, optimized enrichment of palmitoylated proteins, LC-MS/MS analysis, database searching and analysis, and customized bioinformatics analysis.

Protein PTMs are one of the major biological pathways regulating many biological processes and diseases. Our MS-based proteomic analysis of protein palmitoylation enables the identification and analysis of palmitoylated proteins in a wide range of eukaryotic and prokaryotic samples. Please feel free to contact us for more information. We are always ready to assist you!
References
Our products and services are for research use only.