
- Home
- PTMs Proteomics
- Post-Translational Modification of Protein Biopharmaceuticals
- Protein PEGylation Analysis
Protein PEGylation, a cornerstone in the realm of bioconjugation, involves the covalent attachment of polyethylene glycol (PEG) chains to proteins. This transformative modification enhances the therapeutic properties of proteins, offering improved stability, solubility, and pharmacokinetics. A comprehensive analysis of Protein PEGylation is crucial for understanding its implications in biopharmaceuticals and therapeutics.
Post-translational modifications (PTMs) play a pivotal role in the functional diversity of proteins. Protein PEGylation stands as a prominent PTM, altering the native structure and function of proteins to achieve specific therapeutic goals. Precise characterization of PEGylation sites and patterns is imperative for comprehending the impact of PEG on protein structure and function. Advanced analytical methods have become indispensable in elucidating the intricacies of these modifications.
Protein PEGylation analysis stands as a linchpin in the landscape of biopharmaceuticals, shaping the future of therapeutic interventions. As analytical methodologies continue to evolve, the intricate details of PEGylation will unfold, paving the way for a new era of precision medicine and targeted therapeutic solutions. Creative Proteomics has rich research experience and advanced experimental platform in protein PTMs analysis, if you are interested in us, please feel free to contact us.
Supramolecular PEGylation of camptothecin for cancer therapy.
Journal: Materials Today Nano
Published: 2021
Background
PEGylation of anticancer drugs has been used as a first-line technology due to its enhanced pharmacological properties and well-defined molecular structure, enabling industrial production and clinical translation. However, in practice, self-assembled nanomedicines are not therapeutically effective enough due to premature disintegration induced by critical micelle concentration (CMC). An effective way to overcome this premature disintegration is to stabilize the structure by chemical cross-linking to prepare amphiphiles with ultra-low CMC. The host-guest motifs have specific, oriented and reversible noncovalent molecular recognition properties, and thus are widely applicable to the logical construction of multifunctional supramolecular amphiphiles. β-Cyclodextrin (β-CD) and adamantane (AD) have been widely used for the construction of noncovalent couplings and their intelligent self-assembly. In addition, many drug delivery systems based on β-CD and AD motifs have been prepared in recent years and have shown effective therapeutic effects.
Results
The authors propose a rational design of amphiphilic supramolecular PEGylation drugs (Figure 1). The approach is as follows: (1) synthesize β-CD-modified PEG and AD-modified drugs and self-assemble them into nanoparticles by using the host-guest motifs of β-CD and AD as a bridge to construct amphiphilic PEGylation drugs. (2) introduce multifunctional groups in β-CD-modified PEG for cross-linking and tumor microenvironment-sensitive bonding for stimulating the release of reactive drugs. (3) stabilizing the nanostructures by using cross-linking agents to react with β-CD-modified PEG so that the AD-modified anticancer drugs retain their molecular structures.
PEGylation of mono-tetraethylenepentamine β-cyclodextrin (mPEG-TEPA-CD) and adamantane-modified Schiff base (AD-SS-CPT) were first synthesized. The β-CD and AD complexation between them led to the supramolecular polyethylene glycolization of CPT, which could further self-assemble into micelles in aqueous media due to all its amphiphilic groups, as well as by cross-linking the secondary amine on the TEPA chain segments with redox-responsive diacrylate cross-linkers.
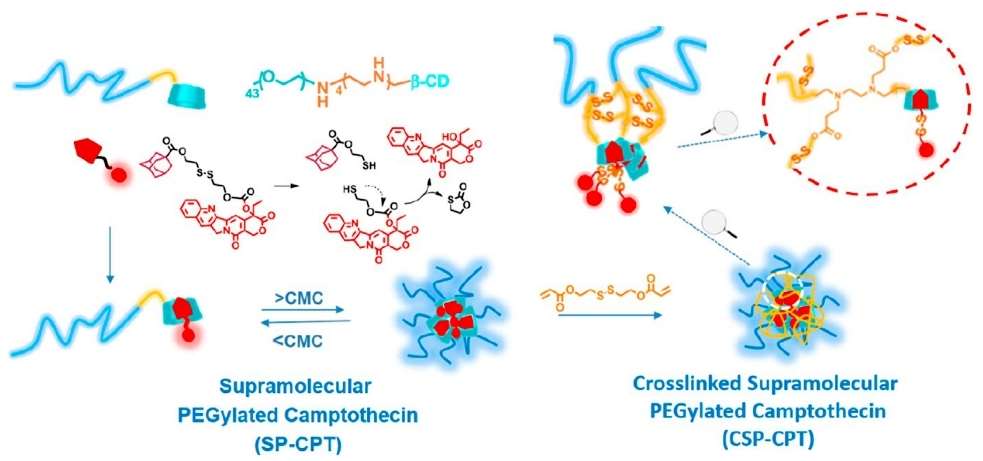 Figure 1
Figure 1
The team characterized the synthesized AD-SS-CPT and mPEG-TEPA-CD. AD-SS-CPT was fully characterized by NMR spectroscopy, ESI-MS spectroscopy and HPLC, indicating that AD-SS-CPT has a specific chemical structure and was synthesized with high purity accurately. The NMR spectra of mPEG-TEPA-CD are shown in Fig. 3d, and the MALDI-TOF MS showed a characteristic increase in molecular weight and an increase in molecular weight with respect to mPEG-Tosyl and TEPA-CD, compared with mPEG-TEPA-CD. A characteristic increase in molecular weight of mPEG-TEPA-CD and a single distribution of peaks corresponding to mPEG-TEPA-CD and mPEG-Tosyl (Figure 2).
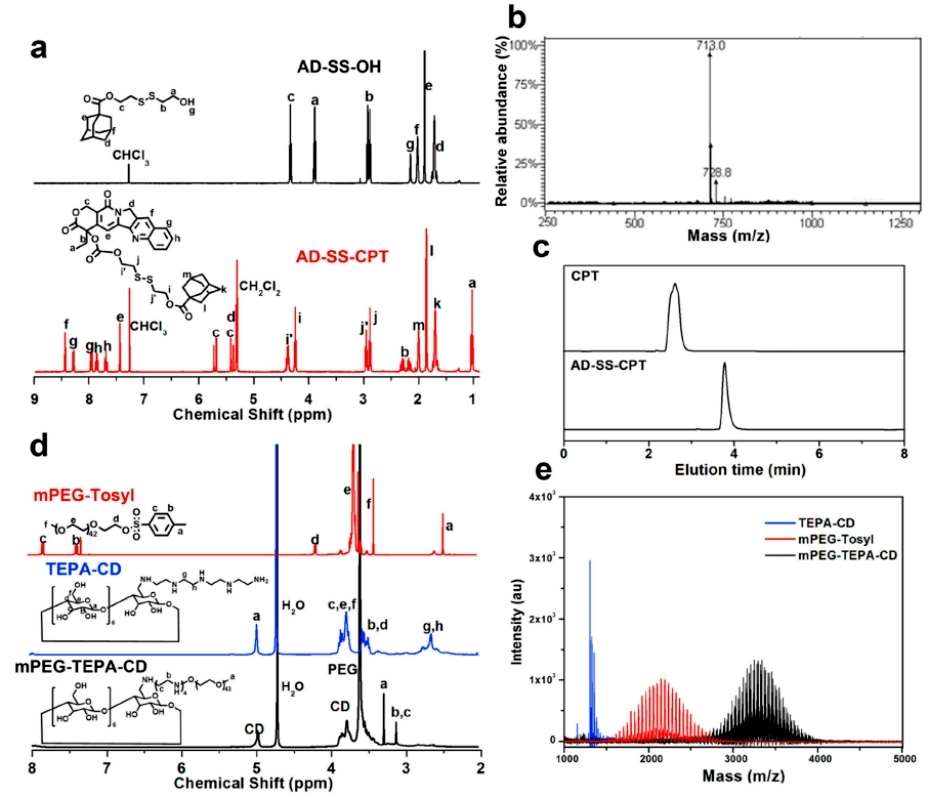 Figure 2
Figure 2
The figure below demonstrates the interaction of mPEG-TEPA-CD with AD-SS-CPT to form a CPT of supramolecular PEGylation.The team characterized the complexation behavior between the two substances by UV-Vis spectroscopic measurements, indicating enhanced solubility of AD-SS-CPT. The study showed good fitting linearity according to the Hildebrande-Benesi equation, indicating a stoichiometry of 1:1 for AD-SS-CPT and mPEG-TEPA-CD, and calculation of the entrapment constants between them by slope/intercept showed that interactions between CPT and β-CD do not affect the PEGylation of CPT. supramolecular self-assembly of PEGylation CPT in aqueous media has been confirmed by the Tyndall effect. Micelles with spherical morphology were confirmed by DLS and TEM. The self-assembled micelles from supramolecular PEGylation CPT are denoted as SP-CPT.To reduce the relatively high CMC value of SP-CPT, SP-CPT was further stabilized by a cross-linking reaction between 2,2′-dithiobisethanol diacrylate and the sec-amino group on the chain segment of TEPA (denoted as CSP-CPT). This suggests that the cross-linking reaction does not affect the chemical structure of AD-SS-CPT. CPT chemical structure, thus providing a well-defined medicinal chemistry component in the crosslinked nanomedicine. Whereas, CSP-CPT has a smaller diameter as compared to SP-CPT because its self-assembly is much tighter(Figure 3).
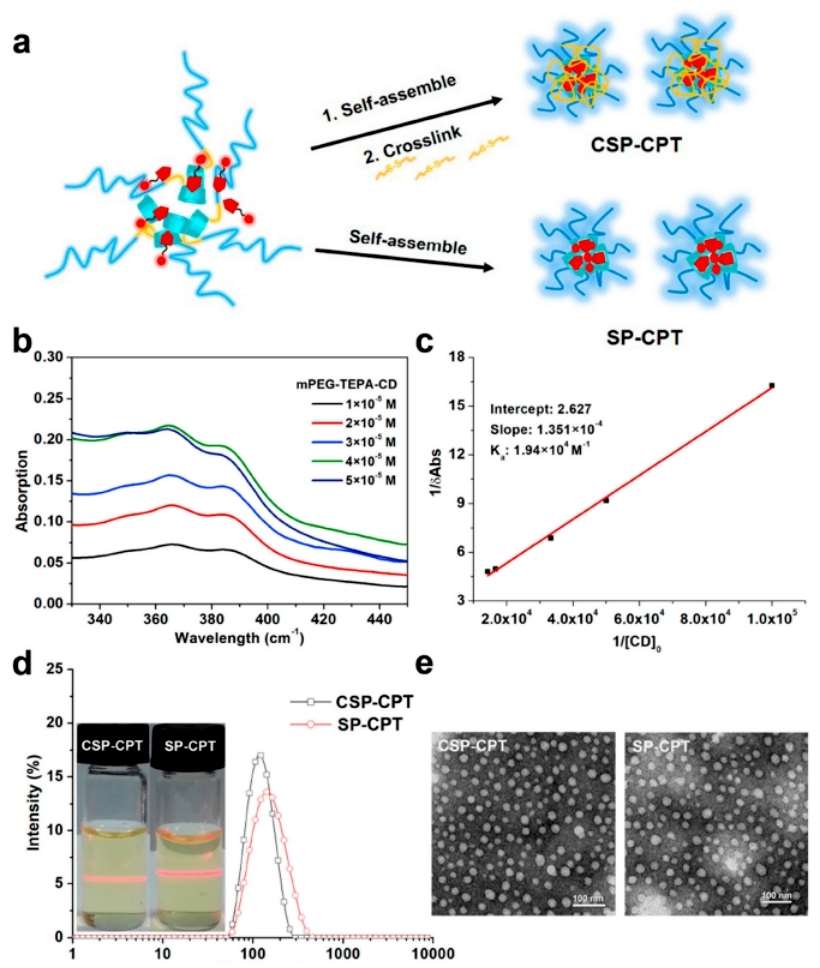 Figure 3
Figure 3
In vivo pharmacokinetic studies in mice showed that CSP-CPT micelles had a longer circulation time than irinotecan. As shown in the figure below, the CSP-CPT micelles were shown to be less cytotoxic than irinotecan when compared to irinotecan. Co-staining of IR 780 into micelles IR 780 CSP-CPT to study the real-time distribution of CSP-CPT in live mice demonstrated that CSP-CPT has a prolonged circulation time and effectively accumulates at the tumor site. The accumulation of IR 780-CSP-CPT in tumors was further quantified by measuring the fluorescence intensity of the tumor region of interest at different imaging times. These results indicate that CSP-CPT has good blood circulation and tumor accumulation properties and can stay at the tumor site for a long time, making CSP-CPT a promising anticancer candidate (Figure 4).
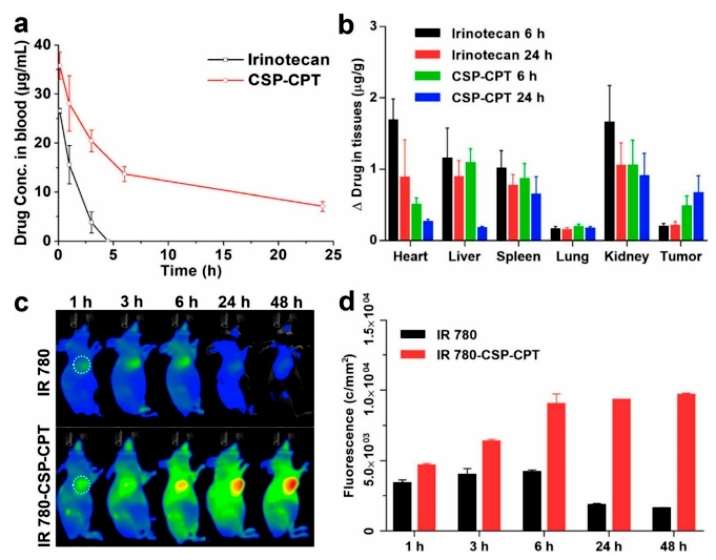 Figure 4
Figure 4
The team used MCF-7 hormonal mice to study the anti-tumor activity of CSP-CPT. As shown in Figure 8a, CSP-CPT micelle treatment effectively inhibited tumor growth and showed complete tumor suppression compared to irinotecan. Changes in body weight of the mice were monitored for 24 days and no significant weight loss was observed. These results were consistent with the circulatory properties and biodistribution of the micelles. At the end of antitumor potency, tumors were isolated and weighed. Mice treated with micelles carrying minimal tumors had an IRT of 94.9%, showing a significantly better IRT than those treated with irinotecan. Histological analysis showed that in the CSP-CPT-treated group, the normal structure of the tumor cells was lost, exhibiting vacuolization and typical apoptotic features. Finally, subacute toxicity evaluation of CSP-CPT was performed to demonstrate the low toxicity of supramolecular PEGylated CPT nanomedicine (Figure 5).
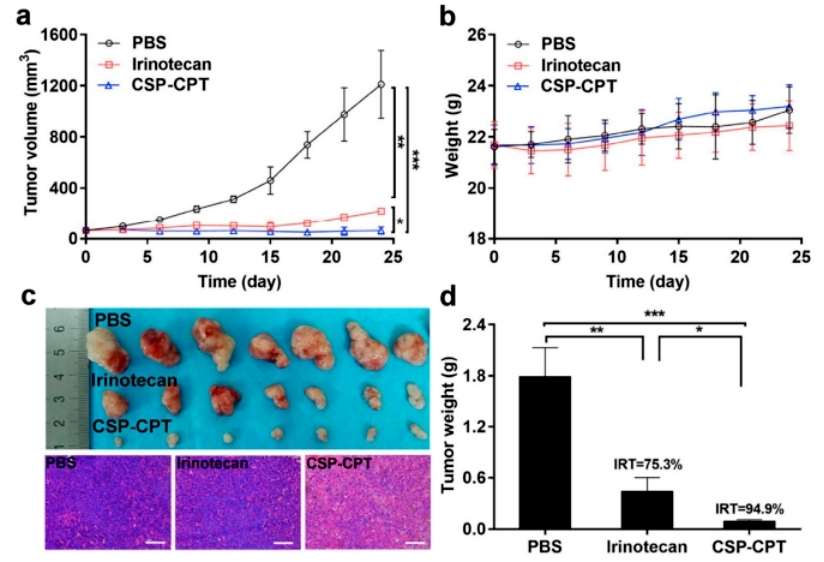 Figure 5
Figure 5
Conclusion
In summary, an amphiphilic PEGylation camptothecin host-guest motif system was prepared from mPEG-TEPA-CD and AD-SS-CPT and self-assembled into micelles in aqueous solution. The micelles were further crosslinked by the addition of a diacrylate cross-linker reacting with the amine groups on the chain segments of mPEG-TEPA-CD and keeping AD-SS-CPT unchanged. When stored at room temperature and in diluted samples, the nanomedicine showed excellent stability, as well as good blood circulation and tumor accumulation, displaying significantly higher therapeutic activity compared to irinotecan. This supramolecular PEGylated camptothecin nanomedicine with stimuli-responsive cross-linking structure, defined molecular prodrug structure, and reproducible drug loading provides a new paradigm for the preparation of nanomedicines for clinical use.
Reference
Our products and services are for research use only.