
- Home
- PTMs Proteomics
- Proteomics Analysis of Crotonylation
Creative Proteomics is a leading provider of custom protein post-translational modifications (PTMs). We are dedicated to providing a series of protein PTM analysis services, including protein crotonylation analysis. We can help our global customers to identify, analyze, and annotate protein crotonylation in a variety of complex biological samples. Lysine crotonylation is a newly discovered acylation modification and can occur in histones and non-histone proteins.
Protein crotonylation occurs mainly on the ε-amino group of lysine in histones. Since the first discovery of lysine crotonylation in histones of mouse sperm and human cell lines, lysine crotonylation has attracted the attention of researchers. To date, there are thousands of crotonylation sites have been identified in proteins. Lysine crotonylation has been confirmed in animals, plants, and various microorganisms (e.g. bacteria and fungi). Crotonylation is a modification closely related to acetylation (an acetylation modification has been well characterized), and it has been reported that histone crotonylation not only has a similar structure but also shares the same set of enzyme systems as histone acetylation. Crotonylation has been disclosed to be involved in chromatin reorganization, nucleotide metabolism, RNA processing, protein activity and localization regulation, and other physiological processes. In addition, crotonylation has been linked to a myriad of diseases such as depression, HIV latency, and cancer.
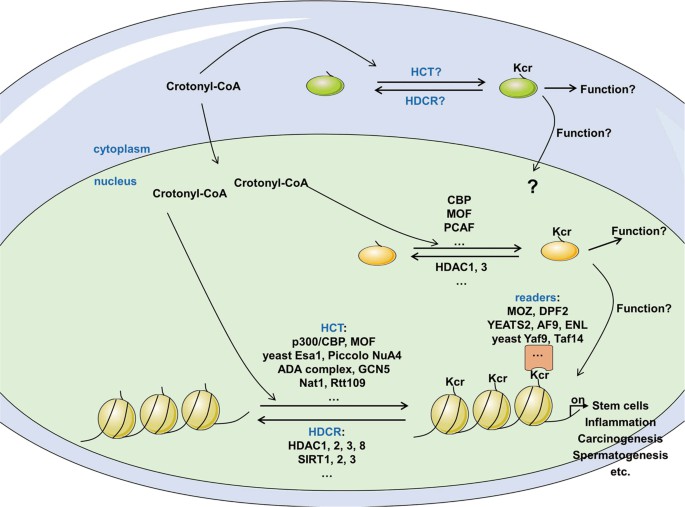 Fig. 1 The modulation of protein crotonylation. (Jiang, Gaoyue, et al., 2021)
Fig. 1 The modulation of protein crotonylation. (Jiang, Gaoyue, et al., 2021)
Lysine crotonylation demonstrates a crucial role in a wide range of biological processes and may be critically implicated in the pathogenesis of diseases. We are dedicated to helping our clients investigate the role of lysine crotonylation in diseases and the underlying mechanism. Our PTM analysis platform is based on mainstream peptide enrichment strategies and high-resolution mass spectrometry (MS). According to the specific PTM, our experienced experts use methods with high specificity and efficient enrichment. Our protein crotonylation analysis service allows the identification and quantification of thousands of lysine crotonylation sites, enabling the whole-proteome characterization of lysine crotonylation. Specifically, our services include the following lists:
Crotonylated proteins have been reported to play important roles in the regulation of various biological processes and the pathogenesis of different diseases. If you have questions about our services, please don't hesitate to contact us for more information. We will arrange for professional staff to contact you as soon as possible!
References
Our products and services are for research use only.