
- Home
- PTMs Proteomics
- Proteomics Analysis of Polyglutamylation
Polyglutamylation, a post-translational modification (PTM) involving the addition of multiple glutamate residues to proteins, has emerged as a crucial regulatory mechanism in cellular processes. This intricate modification plays a pivotal role in modulating protein functions, cellular structures, and ultimately, organismal homeostasis.
Polyglutamylation is characterized by the enzymatic addition of glutamate units to target proteins, typically forming branched structures. This modification is catalyzed by polyglutamylases, a family of enzymes that exhibit substrate specificity for a diverse range of proteins. The reversible nature of polyglutamylation, regulated by deglutamylases, adds an additional layer of complexity to its dynamic regulatory functions.
Accurate analysis of polyglutamylation is imperative for unraveling its functional significance and molecular intricacies. Various cutting-edge analytical methods have been developed to dissect and quantify polyglutamylation patterns with high precision.
Understanding the functional implications of polyglutamylation has broadened its applications across various biological contexts. The intricate interplay between polyglutamylation and cellular processes highlights its significance in health and disease.
Polyglutamylation stands as a dynamic and intricate post-translational modification with far-reaching implications in cellular physiology and disease. The ongoing advancements in analytical methods and the expanding understanding of its functional roles underscore the importance of polyglutamylation as a regulatory mechanism. Unraveling the complexities of polyglutamylation paves the way for innovative therapeutic strategies and enhances our comprehension of fundamental cellular processes. Creative Proteomics relies on advanced analysis techniques, continue to delve deeper of polyglutamylation.
Tubulin engineering by semi-synthesis reveals that polyglutamylation directs detyrosination
Journal: Nat Chem
Published: 2023
Background
Microtubules are dynamic polymers composed of α/β-microtubulin heterodimers that form a dense intracellular network essential for intracellular transport, cell division, motility, and polarization. Post-translational modifications of microtubule protein heterodimers, which enable microtubules to perform specific cellular functions, are mainly focused on unstructured microtubule protein C-terminal tails (CTTs), such as polyglutamylation and de-glycosylation. These PTMs regulate cellular functions, and their dysregulation has been associated with developmental defects and neurodegenerative diseases, making a detailed understanding of the molecular functions of microtubule protein PTMs essential. Microtubulin dimers are high molecular weight and enzyme-sensitive complexes that are difficult to express and purify. Microtubule proteins isolated directly from natural sources (bovine brain) are complex mixtures containing different modifications. They cannot be used to study individual PTMs, so there is an urgent need for a method to produce homogeneous microtubules with precisely mounted and chemically defined PTMs. In this paper, the authors report a semi-synthetic method that enables the introduction of PTMs of interest into microtubulin CTTs, contributing to a particular molecular resolution of the microtubulin code.
Results
To maintain the full function of microtubulin, the authors recombinantly expressed the N-terminal structural domain of α-microtubulin, chemically synthesized the CTT portion containing specific post-translational modifications, and then rapidly ligated the CTT and recombinant proteins using a break protein intron splicing-based technique. This was done by first ligating the IntN of the split intein to the α-microtubulin using a transketolase (engineered sorting enzyme eSrt2A); then IntC-CTT was added, and the IntN and IntC were ligated and the intein spliced out of the protein sequence. The resulting microtubulin sequence is nearly natural, with only the insertion of a cysteine at the splice site, which is necessary to improve splicing efficiency, and a mutation (Val440F). By characterization, the authors produced a fully functional heterodimer of microtubulin that retained all the dynamic and structural properties, allowing a detailed study of the molecular function of the microtubulin code(Figure 1).
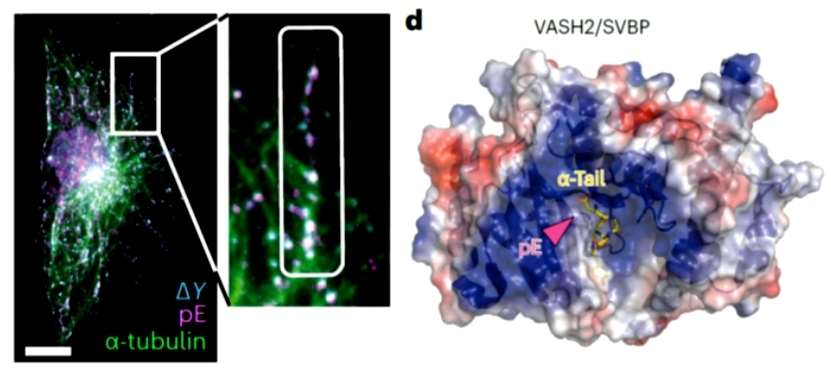 Figure 1
Figure 1
The authors then performed experiments with immunofluorescence imaging in order to investigate whether crosstalk between polyglutamylation and de-tyrosylation occurs in microtubules, and found that polyglutamylation and de-tyrosylation exhibit strong co-localization. In addition, the authors found that the carboxypeptidase vasohibin/SVBP, which mediates de-tyrosylation, exhibits a positive charge around the active site and that an increase in the negative charge in the tail of the microtubule proteins after polyglutamylation modulates this interaction, and therefore the authors decided to test this hypothesis with semi-synthetic polyglutamylated microtubules. The authors synthesized polyglutamylated microtubules of different lengths (α1B, α1B-E5 and α1B-E10), and after stimulating the polymerization of the microtubules and the addition of vasohibin/SVBP, their catalytic activity was found to be highly sensitive to the polyglutamylated state of the substrate microtubules(Figure 2).
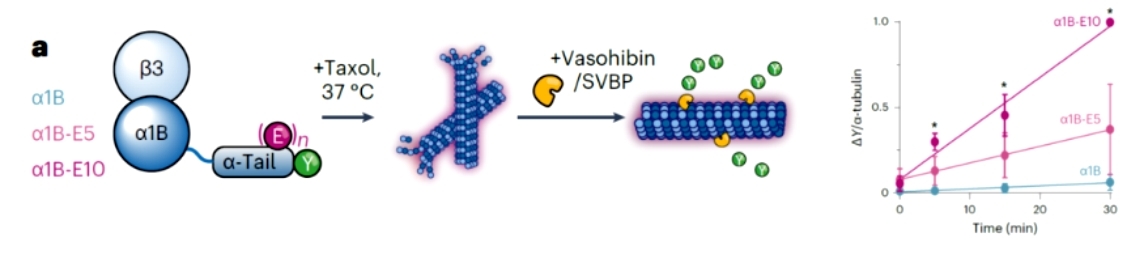 Figure 2
Figure 2
Conclusion
The authors did this by preparing fully functional microtubulin carrying a precisely defined PTM in its C-terminal tail. The authors ligate synthetic α-tubulin tails-which are site-specifically glutamylated-to recombinant human tubulin heterodimers by applying a sortase- and intein-mediated tandem transamidation strategy. Using microtubules reconstituted with these designer tubulins, we find that α-tubulin polyglutamylation promotes its detyrosination by enhancing the activity of the tubulin tyrosine carboxypeptidase vasohibin/small vasohibin-binding protein in a manner dependent on the length of polyglutamyl chains. We also find that modulating polyglutamylation levels in cells results in corresponding changes in detyrosination, corroborating the link between the detyrosination cycle to polyglutamylation. In conclusion, this paper reports a PTM-precisely defined microtubule production strategy and demonstrates a crosstalk mechanism between two key microtubule protein PTMs.
Reference
Our products and services are for research use only.