
- Home
- PTMs Proteomics
- Proteomics Analysis of Hypusine Formation
In the intricate realm of cellular biology, the formation of hypusine emerges as a pivotal process, orchestrating cellular functions at a molecular level. Hypusine, a unique amino acid derivative, plays an indispensable role in the regulation of eukaryotic initiation factor 5A (eIF5A), a factor central to protein synthesis.
At the crux of Hypusine Formation lies its association with eIF5A, a translation factor crucial for peptide bond formation. The post-translational modification of eIF5A involves the conversion of a lysine residue to hypusine, rendering it biologically active. This modification is imperative for the functional maturation of eIF5A, allowing it to partake in essential cellular processes such as mRNA translation elongation and termination.
Beyond its role in protein synthesis, hypusine-modified eIF5A exhibits multifaceted functions. Studies suggest its involvement in cellular proliferation, with implications for cancer biology. The interplay between hypusination and cell cycle progression underscores the intricate regulatory network governing fundamental cellular events.
Hypusine Formation stands as a fascinating and intricately regulated process with far-reaching implications in cellular biology and disease. Creative Proteomics' integration of advanced analytical methods, coupled with our keen focus on their diverse roles and applications, puts the study of prenyl pyrimidine formation at the forefront of contemporary biological research, positions the study of hypusine formation at the forefront of contemporary biological research. As we delve deeper into its complexities, the doors to innovative therapeutic strategies and a more profound understanding of cellular dynamics swing wide open.
Blockade of EIF5A hypusination limits colorectal cancer growth by inhibiting MYC elongation.
Journal: Cell Death Dis
Published: 2020
Background
Colorectal cancer (CRC), the third deadliest and fourth most commonly diagnosed cancer in the world, usually begins as a benign adenomatous polyp, progresses to an advanced adenoma with highly atypical hyperplasia, and ultimately to invasive carcinoma. In the early stages of the disease, the cancer cells are confined to the colon wall and the disease can be cured by surgical removal. As the cancer progresses, the cancer cells pass through the colon wall to the regional lymph nodes, at which point a combination of surgery and adjuvant chemotherapy can cure about 70% of CRC cases. When the cancer reaches an advanced stage, the cancer cells metastasize from in situ to distal, resulting in a worse prognosis for patients, with an average survival rate of only 25-30 months for patients with current treatments. Despite some advances in the molecular mechanisms associated with CRC, the current treatment regimen consists of only a few drugs with limited efficacy, and the overall survival of patients remains unsatisfactory. Therefore, it is particularly important to identify potential therapeutic targets for this disease.
EIF5A is a translation initiation factor regulated by hypusination, a unique post-translational modification catalyzed by DHPS and DOHH initiated by the polyamine spermine. Recent data suggest that hyposinated EIF5A regulates a range of critical cellular processes such as autophagy, senescence, polyamine homeostasis, energy metabolism, and plays a role in cancer. However, the role of inhibiting EIF5A in preclinical cancer models, the related molecular mechanisms, and specific translational targets are still poorly understood.
Results
The study investigated the impact of the DHPS inhibitor GC7 on the growth of different human colorectal cancer (CRC) cell lines (HT29, HCT116, SW480, and LoVo) that exhibit distinct molecular alterations commonly found in the disease. The findings, revealed that the drug effectively inhibited cell proliferation and EIF5A hypusination at micromolar doses, with a standard concentration of 100 μM proving highly effective across all tested cells. Additionally, the drug did not significantly alter the intracellular content of three polyamines (PUT, SPD, and SPM) after 72 h of treatment at this concentration. The study also compared these results with the irreversible ODC inhibitor DFMO, which similarly inhibited EIF5A hypusination and tumor cell growth, highlighting the dependence on polyamine metabolism for CRC growth (Figure 1).
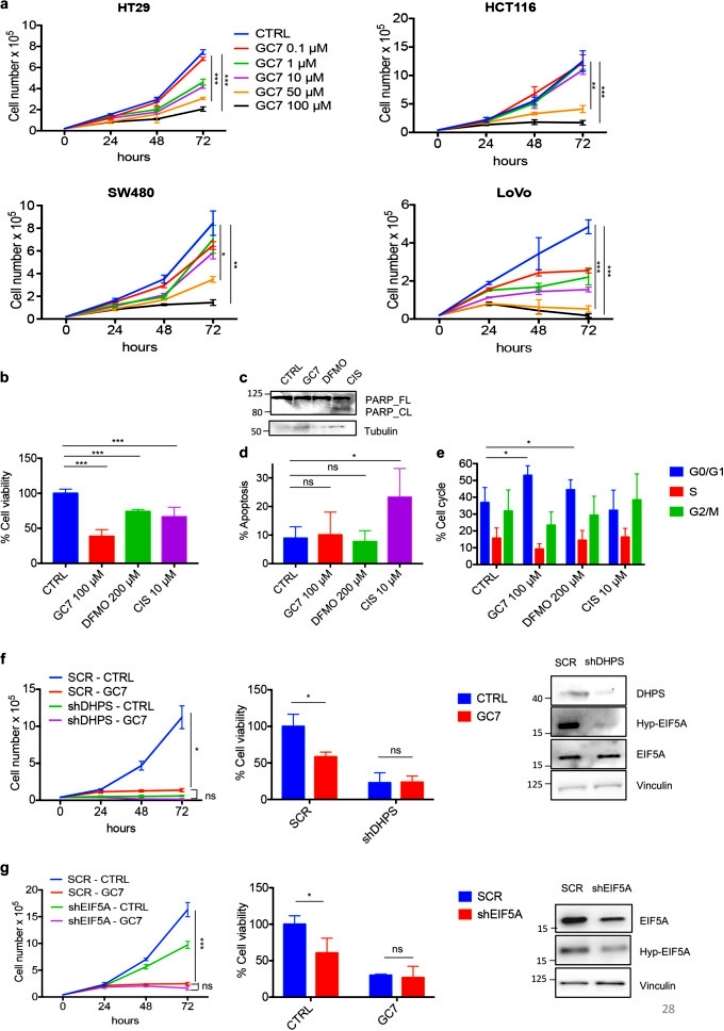 Figure 1
Figure 1
Then, the author found that hypusinated EIF5A could promote the growth of CRC cells by directly regulating the biosynthesis of MYC in specific suspension states. Inhibition of the hypusination effect of EIF5A by the DHPS inhibitor GC7, lentivirus-mediated knockdown of DHPS or EIF5A inhibited the growth of CRC cells (Figure 2).
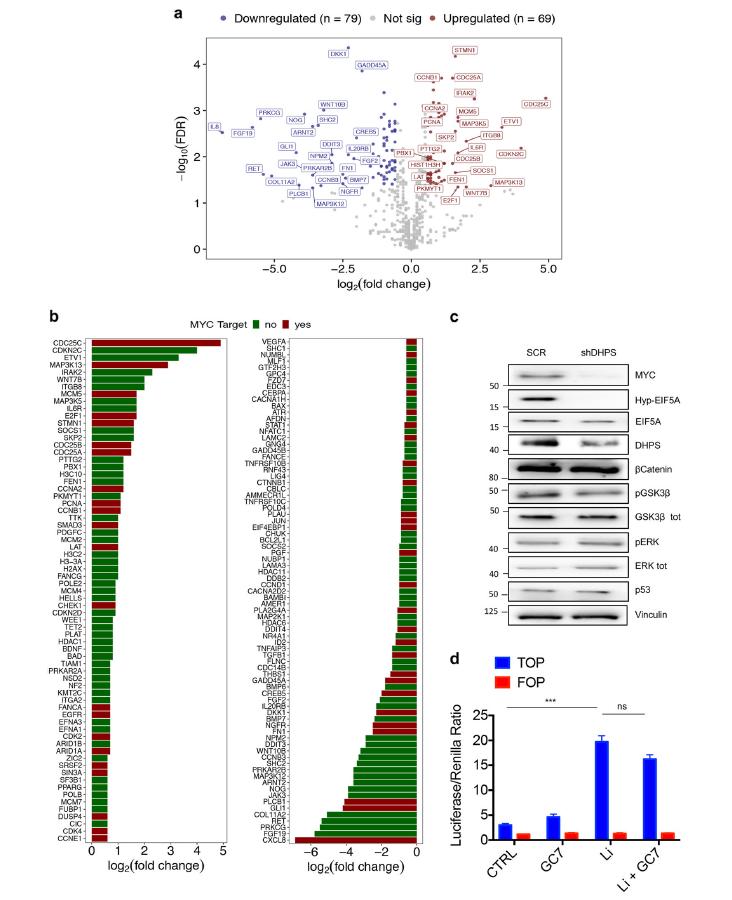 Figure 2
Figure 2
Multigene expression analysis revealed that inhibition of hypusination action impairs the expression of MYC-regulated transcripts. The researchers further demonstrated that EIF5A regulates MYC synthesis primarily by attenuating the ribosomal arresting effects of five different pause motifs in the MYC CDS without affecting its mRNA levels or protein stability. Notably, the researchers found that blockade of hypusination action in a preclinical model of CRC induced significant growth inhibition and was able to significantly reduce polyp size in the APCMin/+ mouse model of familial adenomatous polyposis (FAP) (Figure 3).
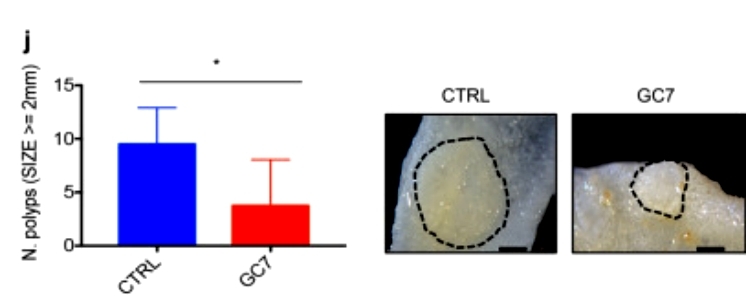 Figure 3
Figure 3
Conclusion
The study reveals that hypusinated EIF5A promotes colorectal cancer (CRC) cell growth by directly regulating MYC biosynthesis at specific pausing motifs. Inhibiting EIF5A hypusination through the DHPS inhibitor GC7 or lentiviral-mediated knockdown of DHPS or EIF5A reduces CRC cell growth. Multiplex gene expression analysis indicates that hypusination inhibition impairs transcripts regulated by MYC, suggesting the involvement of this oncogene. Furthermore, blockade of the hypusination axis exhibits a significant growth inhibitory effect in preclinical CRC models and reduces polyp size in APCMin/+ mice, a model of familial adenomatous polyposis (FAP). These findings unveil a novel mechanism linking the tumor-promoting properties of hypusinated EIF5A to its ability to regulate MYC elongation, proposing DHPS/EIF5A inhibitors as a rationale for CRC therapy.
Reference
Our products and services are for research use only.