
- Home
- PTMs Proteomics
- Proteomics Analysis of Citrullination
Citrullination, a post-translational modification (PTM), involves the conversion of arginine residues in proteins to citrulline. This enzymatic process is catalyzed by peptidylarginine deiminases (PADs), a family of calcium-dependent enzymes. While originally identified in the context of autoimmune diseases such as rheumatoid arthritis, citrullination has emerged as a crucial cellular mechanism with implications beyond autoimmunity.
Citrullination, once confined to the realm of autoimmune diseases, has evolved into a multifaceted player in cellular processes. From epigenetic regulation to disease biomarkers and drug development, the implications of citrullination are far-reaching. Creative Proteomics is dedicated to protein research, we utilize various analytical methods to help researches understand the citrullination's intricacies.
Citrullinated fibrinogen-SAAs complex causes vascular metastagenesis
Journal: Nat Commun
Published: 2023
Background
Cancer metastasis refers to the process by which cancerous cells spread from the primary tumor site into other regions of the body, and despite the great strides scientists are making in the field of cancer research today, the drivers that drive the selective spread of cancer to specific regions of the body are still largely unknown to researchers. In this article, researchers have identified one of these potential molecular mechanisms. By elucidating that post-translational citrullination of fibrinogen creates a metastatic habitat at susceptible sites, the researchers may reveal the possibility of detecting metastatic predictive sites, where lung endothelial cells mediate the citrullination of fibrinogen, which alters its conformation, surface charge, and binding properties associated with serum amyloid A or SAAs. SAAs, thus enabling them to become host tissue-derived metastatic pathogens.
Results
Citrullination is the process by which arginine in proteins is converted to citrulline; in this study, the researchers focused on specific modifications that occur in the protein fibrinogen, which contains one or more carbohydrate chains and plays a key role in the blood clotting process. To further elucidate the mechanisms of metastasis in lung cancer, after establishing a direct correlation between dispersed fibrinogen deposits in lung blood vessels and an increased likelihood of cancer metastasis, the researchers decided to scrutinize the biochemical modifications that occur after fibrinogen is synthesized (i.e., translated) in vivo, starting with dissected lung tissue samples obtained from individuals with and without cancer.
When stained with the dye, there was a significant increase in fibrinogen-positive areas in the lung tissue of cancer patients compared to those who were cancer-free at the time of death, and subsequent analyses showed that the expression of the serum amyloid A gene (SAA, a known pre-metastatic gene in the mouse organism) was five times higher in the cancer patients than in the bodies of individuals who did not have cancer. Based on this, the researchers then developed mice with humanized SAAs, i.e., rodents genetically programmed to produce a compatible version of the human SAA protein, and, unsurprisingly, metastasis of lung tumors was more severe in mice with humanized SAA clusters compared to mice with the SAA cluster gene blocked. Next, the researchers investigated the interaction between SAA3 proteins and fibrinogen in the lungs of tumor-bearing mice. During the course of their experiments, the researchers found that SAAs mediate the citrullination of fibrinogen and convert the standard amino acid arginine to the non-standard citrulline(Figure 1).
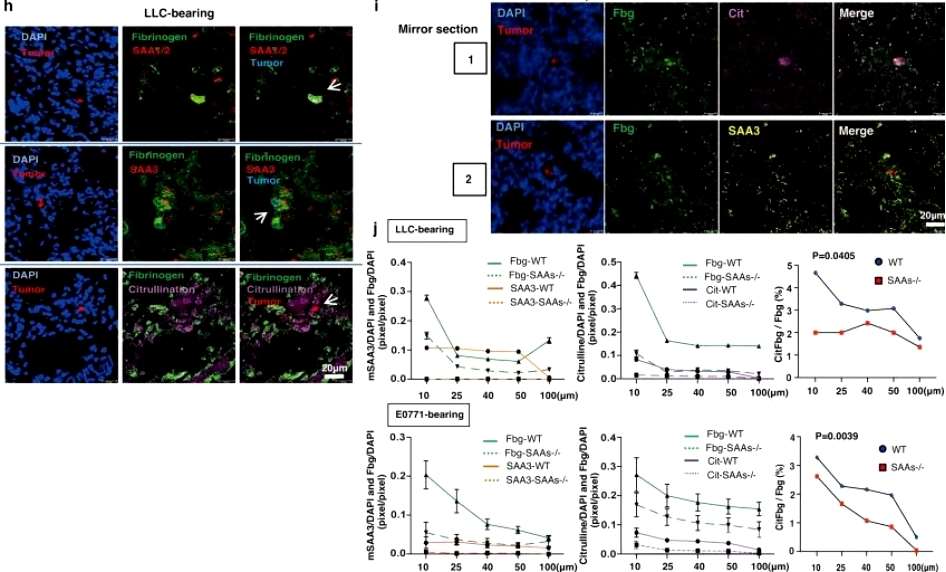 (Figure 1)
(Figure 1)
Further experimental results showed that SAAs-mediated citrullination of fibrinogen leads to tumor metastasis in the lungs in a mouse model, and finally, a protein complex containing citrullinated fibrinogen and SAAs (SAAs-CitFbg) is apparently recruiting cancer cells and can promote metastasis in lung tumors in a mouse model, Based on these results, the current researchers successfully designed a novel CitFbg peptide to combat cancer metastasis in a mouse model. Researcher Sachie Hiratsuka added that a CitFbg peptide may be able to act as a competitive inhibitor to block the homing process of metastatic cells to the SAAs-CitFbg sites, and that potential metastatic sites in patients' lungs could be clearly visualized by the CitFbg-specific antibody developed by the researchers(Figure 2).
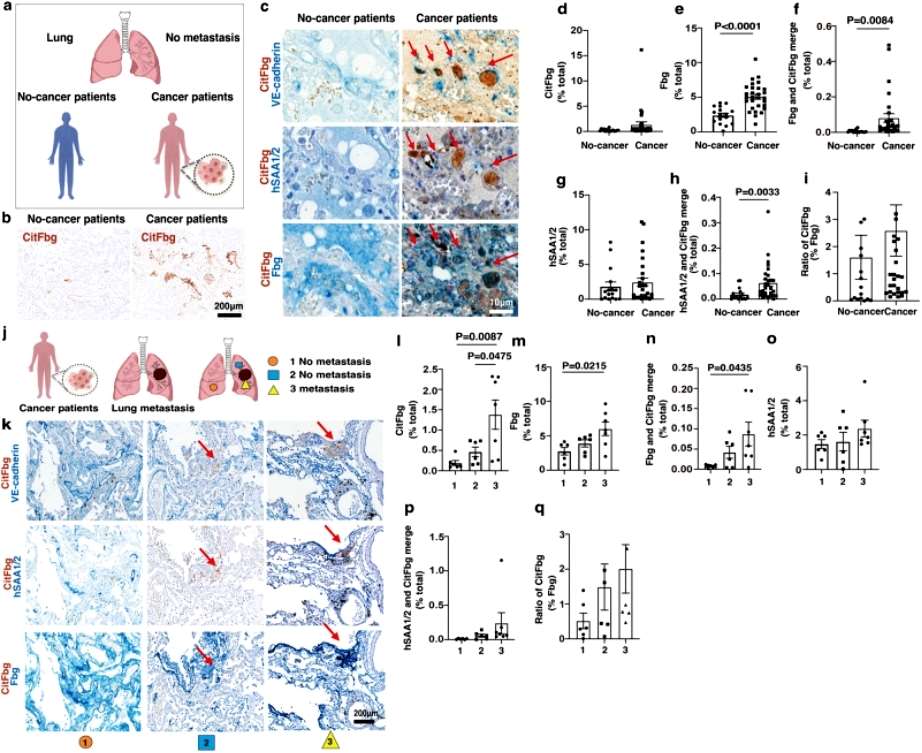 Figure 2
Figure 2
Conclusion
The results of this paper suggest that CitFbg deposition may be associated with an increased risk of metastasis in cancer patients and that citrullinated peptides may be effective in counteracting this in mouse models. The prediction of cancer metastasis may provide researchers with an opportunity to intervene early to mitigate the progression of cancer and the life-threatening risks associated with it, and although more clinical trials are needed, this study may pave the way for the development of novel anti-metastatic drugs in the future. The findings also add significant value to cancer research and may have an impact on the prevention and treatment of cancer with herbal medicines. In summary, the results of this paper suggest that the deposition of CitFbg may indicate the risk of metastasis in cancer patients and that citrullinated peptides may also be expected to serve as a specific class of cancer metastatic inhibitors.
Reference
Our products and services are for research use only.