
- Home
- PTMs Proteomics
- Quantitative Analysis Of Glycation
Protein glycation is a spontaneous post-translational modification (PTM) of proteins found in physiological systems and food products. It involves the non enzymatic covalent attachment of a reducing sugar or sugar derivative to a Protein. Glycation primarily occurs at the α-amino termini of proteins and also at epsilon amino group of the Lys side chain. Glycation is a chemical modification in which susceptible primary amine groups reacts with carbonyl groups to form a highly reactive and unstable Schiff base that undergoes multistep rearrangement called Amadori rearrangement to form ketoamine.The ketoamine formed as a result of glycation is also called as Amadori ketoamine. This Amadori ketoamine contains a more stable covalent bond than the reactive Schiff's base.
The process of glycation is fundamentally a chemical condensation reaction that occurs between the primary amine groups found in amino acid residues of proteins and the aldehyde groups present in reducing sugars. Primary amine groups, known for their high reactivity with carbonyl groups, are commonly encountered in the N-terminus of proteins and in internal Lys residues.
Reducing sugars like glucose (Glc) and galactose (Gal) contain hemiacetal groups that function as aldehyde moieties. Hexose sugars exist in an equilibrium between their open-chain and pyranose ring forms in solution. Consequently, the aldehyde groups within the open-chain form of hexoses exist in equilibrium with hemiacetal groups in solution.
In a basic environment, primary amine groups carry a positive charge, rendering them nucleophilic and allowing them to engage in condensation reactions with the carbonyl groups of reducing sugars. This interaction leads to the formation of reactive Schiff's base (refer to Figure 1). The Schiff's base, characterized by high reactivity and instability, swiftly undergoes Amadori rearrangement (as depicted in Figure 1), ultimately forming the relatively stable Amadori ketoamine functionality (Mori and Manning, 1986). Subsequently, the Amadori ketoamine undergoes degradation into small organic molecules collectively known as Advanced Glycation End Products (AGEs) (Khalifah et al., 1999).
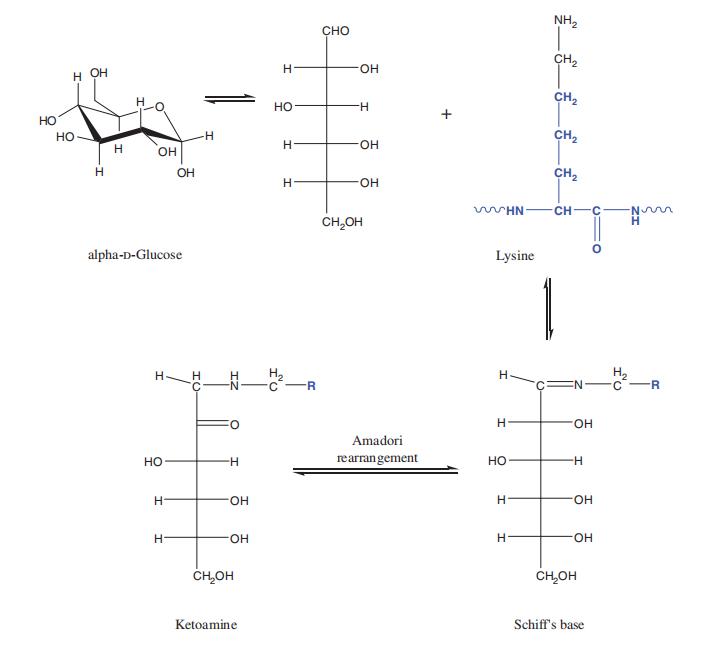 Figure 1 Mechanism of protein glycation.
Figure 1 Mechanism of protein glycation.
Protein glycation has significant clinical utility in metabolic monitoring and diagnosis. When glucose interacts with albumin and hemoglobin, it leads to the formation of glycated derivatives, namely, glycated albumin and glycated hemoglobin. These derivatives serve as essential clinical markers for assessing glycemic control in diabetes, reflecting the glycemic status over different time frames: 3–4 weeks for glycated albumin and 6–8 weeks for glycated hemoglobin.
A specific range of glycated hemoglobin, typically falling within 39–47 mmol/mol Hb, represents an intermediate level between that observed in healthy individuals and diabetes patients. This range serves as a diagnostic indicator for impaired glucose tolerance, preceding the onset of diabetes. Clinically, it plays a crucial role in the screening and diagnosis of prediabetes.
Furthermore, the formation of Advanced Glycation End Products (AGEs), resulting from prolonged glycation processes, is implicated in various health conditions. These conditions encompass aging-related disorders such as age-related macular degeneration and cataracts, obesity, vascular complications associated with diabetes (including nephropathy, retinopathy, and neuropathy), cirrhosis, cardiovascular disease, renal failure, and neurological disorders like Alzheimer's disease and Parkinson's disease. Protein glycation, therefore, has profound clinical implications in understanding and managing a spectrum of health-related conditions.
To study glycated peptides and proteins, mass spectrometry (MS) has emerged as a powerful analytical tool. The detection of fructosamine-modified peptides, such as FL, involves a characteristic mass increment of +162 Da. Electrospray positive ion MS and matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) MS have been instrumental in detecting glycation in intact proteins and large peptide chains.
Scientists have utilized mass spectrometry (MS) methodologies to identify and quantify fructosamine-modified hemoglobin chains, yielding valuable insights into the precise locations of fructosamine attachment. Furthermore, the application of matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) has unveiled noteworthy disparities in the mass increments between glycated human serum albumin (HSA) derived from in vitro preparations and HSA obtained from in vivo plasma specimens.
For MS analysis of glycated peptides, collision-induced dissociation (CID) and higher-energy collisional dissociation (HCD) have been utilized to generate characteristic fragment ions, aiding in fructosamine site localization. Electron transfer dissociation (ETD) fragmentation has proven valuable, offering extensive c- and z-type ions for peptide sequencing and fructosamine site determination.
Moreover, mass spectrometry (MS) has been harnessed for the identification of the degradation product FL and the presence of Advanced Glycation End Product (AGE) CML at identical sites as fructosamine residues within diverse proteins. Furthermore, investigations into peptides glycated by methylglyoxal (MG) have unveiled the existence of hydroimidazolone and dihydroxyimidazolidine residues.
However, it is essential to note that the labile nature of these AGEs may be influenced by preanalytic processing conditions. Tryptic digestion methods, involving prolonged periods of sample incubation at high pH and/or temperature, can lead to the reversal of hydroimidazolone to dihydroxyimidazolidine and deglycation. Therefore, modifications to tryptic digestion techniques are necessary to minimize pH increase and avoid sample heating when studying MG-modified proteins and related post-translational modifications (PTMs).
In summary, mass spectrometry has demonstrated its utility as an invaluable instrument for the examination of glycated peptides. It not only provides crucial information regarding the specific locations of glycation but also contributes significantly to the comprehension of diseases associated with glycation processes.
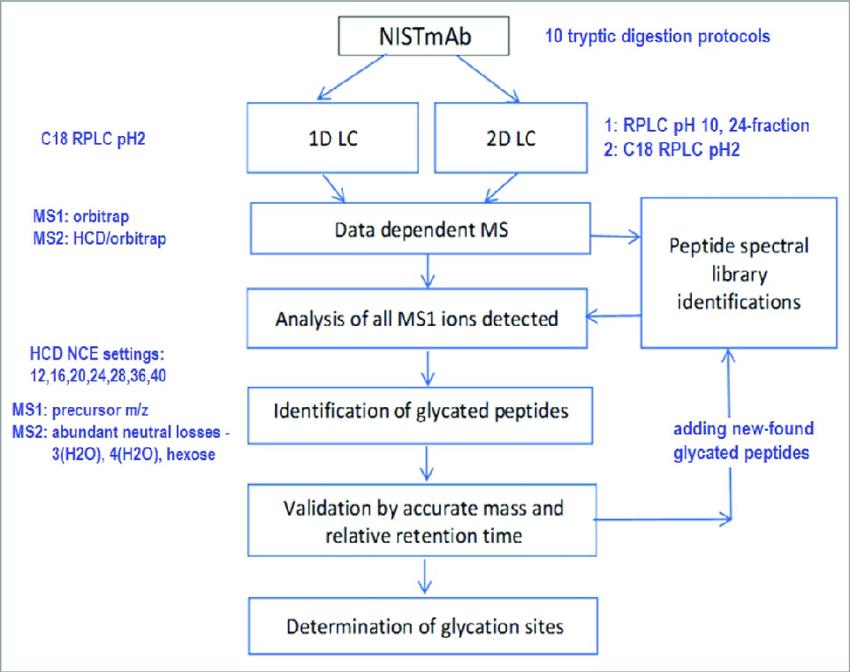 (Qian Dong et et. mAbs 2018)
(Qian Dong et et. mAbs 2018)
Our Advanced Glycation Quantitative Analysis Service is meticulously designed to provide you with comprehensive insights into protein glycation, enabling a deeper understanding of its role in various diseases and physiological processes. We offer a range of specialized services, each tailored to meet your specific research needs:
Customized Preanalytical Sample Processing: Our application of cutting-edge instrumentation guarantees the optimal preparation of your specimens for analysis. This encompasses the implementation of advanced methodologies to manage the relatively fragile nature of glycation, safeguarding the integrity of your samples.
Detection and Quantification of Total Glycation: Within our service, we employ state-of-the-art mass spectrometry technology, encompassing electron transfer dissociation (ETD) and multiple reaction monitoring (MRM). These advanced methodologies significantly enhance the sensitivity, precision, and accuracy of our glycation detection and quantification protocols. Through the utilization of LC-MS/MS and MRM targeting glycated tryptic peptides, we furnish you with meticulous quantitative assessments of glycation levels.
Bioinformatics Analysis: Our commitment to providing reliable results extends to the integration of cutting-edge bioinformatics tools that are currently in development. These tools significantly contribute to the analysis of protein glycation sites, aiding in the interpretation of their functional and physiological implications. By incorporating bioinformatics into our service, we ensure the utmost confidence in the accuracy of your results.
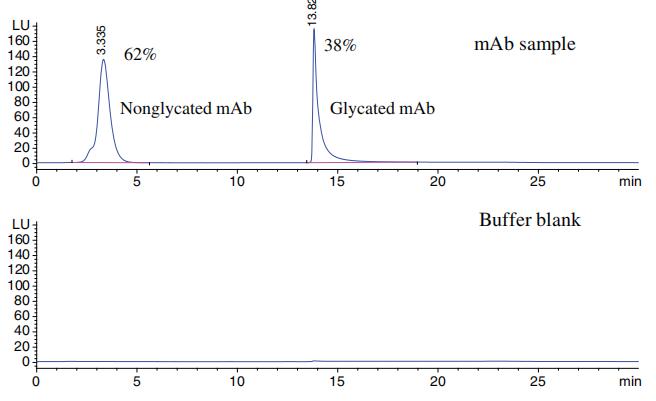 Separation of glycated and non-glycated mAb using boronate affinity chromatography.
Separation of glycated and non-glycated mAb using boronate affinity chromatography.
Customization: Our service is adept at tailoring solutions to your specific research requisites, guaranteeing the fulfillment of your distinct needs.
Cutting-Edge Technology: We leverage state-of-the-art mass spectrometry instrumentation, including electron transfer dissociation (ETD) and multiple reaction monitoring (MRM), to furnish results that are characterized by precision and accuracy.
Experienced Team: Our team of experts boasts extensive experience in the field of glycation analysis, assuring the delivery of service of the utmost quality.
Comprehensive Insights: We offer a comprehensive perspective on protein glycation, spanning from sample preparation to data analysis. This approach allows for an in-depth comprehension of the implications of glycation in your research.
Reliability: Our unwavering commitment to quality and precision instills confidence in the results generated by our service, making them a dependable resource for your research endeavors.
References
Our products and services are for research use only.