
- Home
- PTMs Proteomics
- Post-Translational Modification of Protein Biopharmaceuticals
- Antibody Glycosylation Analysis
Antibodies, pivotal components of the immune system, exhibit remarkable heterogeneity owing to variations in glycosylation patterns. Glycosylation profoundly influences antibody structure, stability, and function, impacting therapeutic efficacy and immunogenicity. In the realm of biopharmaceuticals, understanding and characterizing antibody glycosylation is imperative for ensuring product quality, safety, and efficacy.
Antibody glycosylation analysis is a multifaceted field, intertwining cutting-edge analytical methods with diverse applications in biopharmaceutical development, disease research, and immunogenicity assessment. As the industry navigates the complex landscape of antibody therapeutics, a thorough understanding of glycosylation dynamics emerges as an indispensable tool, guiding the optimization of therapeutic antibodies for improved patient outcomes. Creative Proteomics has rich experience in antibody glycosylation research, if you are interested in us, please feel free to contact us, we look forward to working with you.
Antigen-Specific Antibody Glycosylation Is Regulated via Vaccination
Journal: PLoS Pathog
Published: 2016
Background
The crucial role of non-neutralizing antibody effector functions, such as antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC), in protecting against and controlling various infections, including HIV, influenza, Ebola virus, and bacterial infections. The dominant subclass of antibodies in the blood, IgG1, is known to drive potent, long-lived antibody effector activity. However, despite all individuals producing IgG1 antibodies, there is variability in the protective immunity provided. The IgG antibody glycosylation is a modification that occurs within the constant region (Fc) and significantly influences the inflammatory profile and effector functions of antibodies. Changes in glycosylation, including variations in galactose, sialic acid, fucose, and N-acetylglucosamine, impact the affinity of antibodies for innate immune receptors. The article notes that alterations in antibody glycosylation have been demonstrated in therapeutic settings, affecting therapeutic efficacy and inflammation in conditions such as rheumatoid arthritis.
The central question addressed is whether antibody glycosylation is actively regulated in vivo. The study explores distinct glycoprofiles on antigen- and pathogen-specific antibodies, illustrating unique antibody glycan profiles against individual antigens. The findings suggest that antibody glycosylation is selectively tuned against specific pathogens and antigens, pointing to antigen-specific regulation of antibody glycosylation.
Results
The author discusses the impact of inflammatory diseases and viral infections on the glycosylation patterns of antibodies in the context of specific antigens. The study focused on HIV-infected patients, selectively enriching antibodies for HIV envelope (gp120), HIV capsid (p24), and influenza envelope (HA). Glycan profiling was conducted, revealing distinct glycosylation differences not only between antigen-specific antibodies and bulk circulating antibodies but also among the specific antibodies for the three antigens. Notably, gp120-specific antibodies exhibited elevated levels of agalactosylated glycans, slightly increased fucosylated and bisected glycans, and decreased sialylated structures compared to bulk antibodies. These glycan modifications suggest a more functional and inflammatory glycan profile on gp120-specific antibodies, potentially influencing antibody-dependent cellular cytotoxicity (ADCC) and complement-mediated killing (Figure 1).
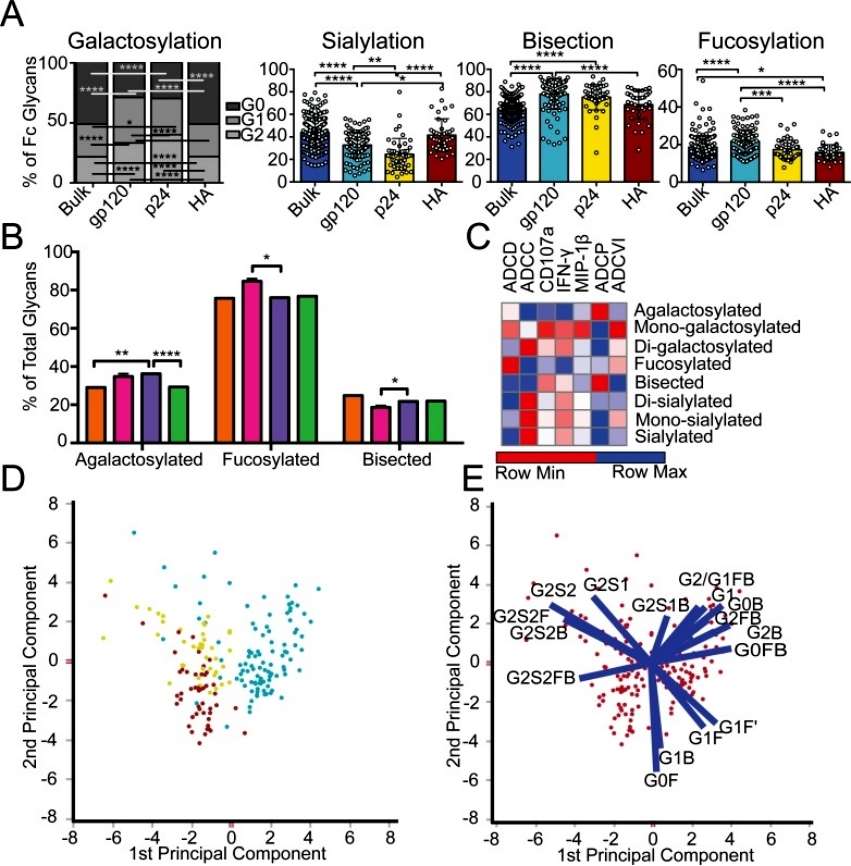 Figure 1
Figure 1
The author explores the programmability and reproducibility of antibody glycosylation through a vaccine trial using the experimental Ad26/Ad35 expressing an HIV Envelop protein A (B003/IPCAVD-004/HVTN091 trial). The trial took place in the United States, Kenya/Rwanda (East Africa), and South Africa. Given the significant differences in bulk IgG Fc glycosylation profiles in inflammatory and infectious diseases, the study initially compared the baseline differences in bulk circulating antibody Fc glycosylation across the three trial sites.
The analysis revealed notable variations in bulk IgG Fc galactosylation and sialylation among individuals in the three regions. Specifically, individuals from both African regions exhibited higher proportions of agalactosylated (G0) Fcs. East Africans, in particular, had the lowest bulk antibody sialylation. The data suggest that bulk antibody glycosylation in Africans is associated with inflammatory glycosylation, with East Africans showing the most inflammatory profile. Since the bulk IgG population consists of various antigen-specific antibodies corresponding to encountered pathogens, these differences may be linked to genetic background, diet, and/or pathogen exposure in each geographical location (Figure 2).
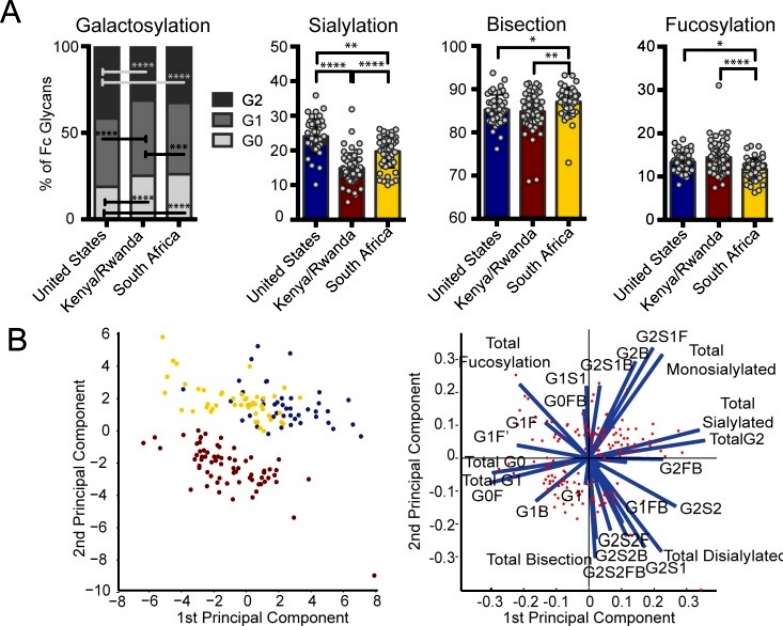 Figure 2
Figure 2
The author aimed to investigate whether antigen-specific antibodies induced through vaccination have distinct glycosylation profiles compared to bulk circulating antibodies. To achieve this, antibodies elicited by the viral vector were enriched from bulk IgG using vaccine-matched gp120 antigens in a subset of vaccinees from each region. Notably, the viral vector-induced antibodies showed significant differences from bulk antibody glycans based on principal component analysis (PCA). There was no overlap in the overall glycan profiles of antigen-specific and bulk antibodies. Moreover, all glycan types were found to be significantly different in vaccine-specific antibodies compared to bulk antibody glycosylation, with vaccine-specific glycans being more galactosylated, mono-galactosylated, sialylated, and bisected, but less fucosylated and di-galactosylated. The separation between antigen-specific and bulk antibodies was primarily driven by differences in fucosylation and sialylation. This analysis demonstrates that antigen-specific antibody glycosylation is specific and influenced by the immune signals delivered during immunization (Figure 3).
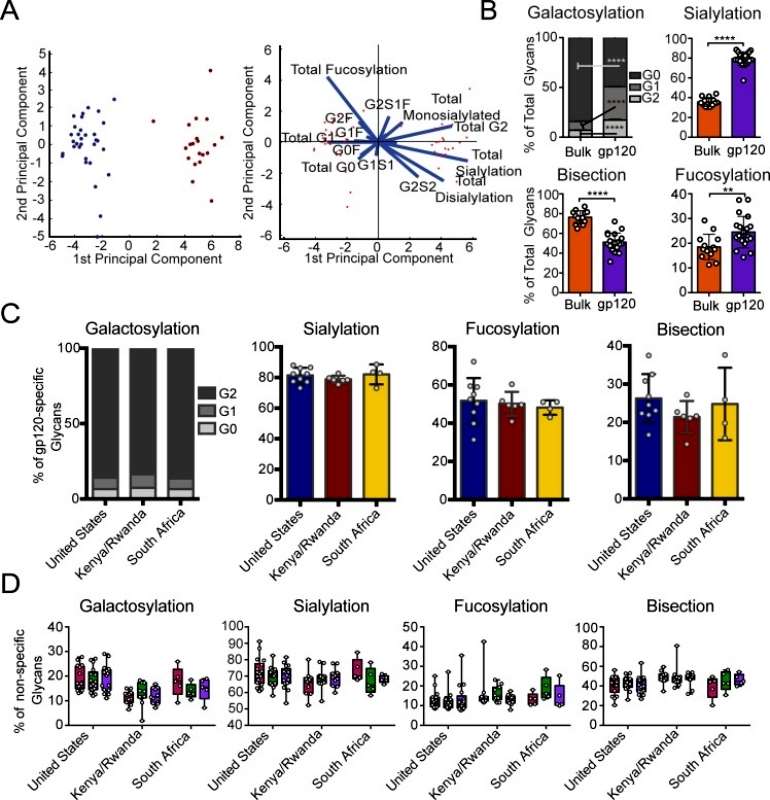 Figure 3
Figure 3
Using principal component analysis (PCA), significant separation of gp120-specific antibody glycosylation profiles was observed across the two vaccines for galactose, sialic acid, and b-GlcNAc. Specifically, the B003/IPCAVD-004/HVTN091 trial induced more anti-inflammatory glycan structures, with higher proportions of di-galactosylated and sialylated glycans compared to the VAX003 trial. The viral vector used in B003/IPCAVD-004/HVTN091 also increased the proportion of bisected glycan structures, known to enhance antibody-dependent cellular cytotoxicity (ADCC) activity and functionality. However, no differences were noted in fucose content between the two vaccine trials. In summary, the viral vector in B003/IPCAVD-004/HVTN091 induced a less inflammatory but highly functional antibody glycan profile compared to the alum-adjuvanted VAX003 study, which exhibited poorly functional but highly inflammatory antibody glycan profiles (Figure 4).
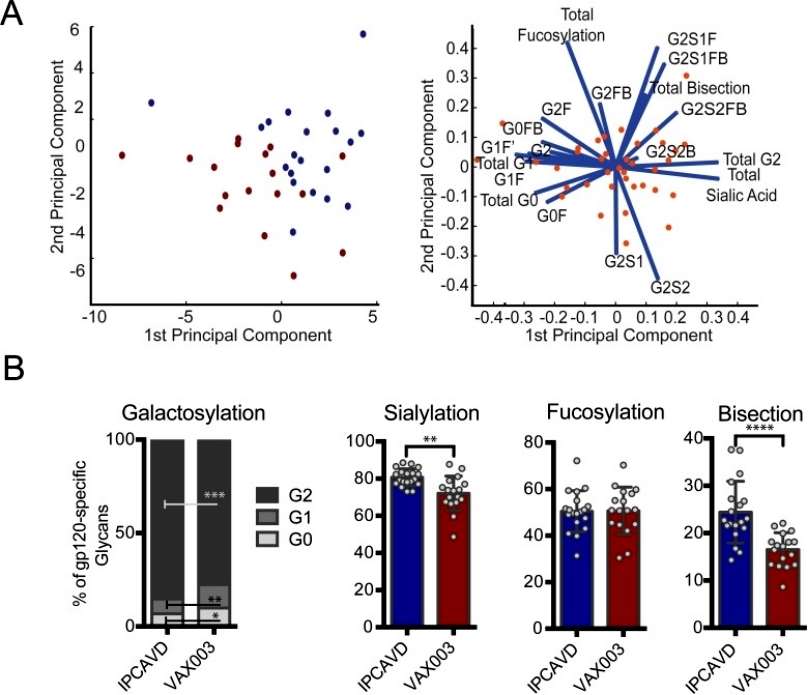 Figure 4
Figure 4
Conclusion
The study demonstrates that antibody glycosylation is influenced by the specific antigen and pathogen during HIV infection. Notably, substantial variations exist in bulk IgG glycosylation among individuals from different geographical locations. However, immunization has the ability to override these differences, leading to the generation of antigen-specific antibodies with similar glycosylation patterns. Furthermore, the research indicates that distinct vaccine regimens result in different antigen-specific IgG glycosylation profiles. This suggests that antibody glycosylation is not only programmable but can be manipulated by delivering specific inflammatory signals during B cell priming. These findings strongly support the idea that the immune system inherently shapes antibody glycosylation in an antigen-specific manner. Moreover, it emphasizes the potential for next-generation therapeutics and vaccines to leverage the antiviral activity of the innate immune system through targeted alterations in antibody glycosylation in vivo.
Reference
Our products and services are for research use only.