
- Home
- PTMs Proteomics
- Post-Translational Modification of Protein Biopharmaceuticals
- Protein Drug Glycosylation Analysis
Protein drugs have revolutionized the field of therapeutics, offering targeted and efficacious treatment options. Glycosylation, a crucial post-translational modification (PTM), significantly influences the biological activity, stability, and pharmacokinetics of these therapeutic proteins. Glycosylation, the enzymatic attachment of sugar moieties to proteins, emerges as a critical determinant in the structure and function of therapeutic proteins. The addition of glycans to protein drugs modulates their solubility, immunogenicity, and receptor binding, thereby influencing their therapeutic efficacy. Understanding the glycosylation profile is imperative for ensuring the safety and efficacy of protein drugs.
N-linked and O-linked glycosylation are predominant forms of protein glycosylation. N-glycans attach to the amide nitrogen of asparagine residues, while O-glycans link to the hydroxyl oxygen of serine or threonine residues. The interplay between these glycosylation types contributes to the three-dimensional structure and functionality of protein drugs. Glycosylation profoundly affects the conformational stability and folding of protein drugs. It influences protein-protein interactions, cellular trafficking, and clearance rates. In therapeutic proteins, such as monoclonal antibodies, the glycosylation pattern can alter the antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) mechanisms, influencing their clinical outcomes.
Accurate and high-throughput analytical methods are essential for deciphering the complex glycosylation patterns of protein drugs. Various techniques have emerged, each offering unique insights into the glycosylation landscape.
Mass Spectrometry (MS)
Capillary Electrophoresis (CE)
Glycan Microarrays
Creative Proteomics is dedicated to glycosylation research, and our integration of advanced analytical methods provides effective solutions in advancing the continued development of this field.
Glycosylation is key for enhancing drug recognition into spike glycoprotein of SARS-CoV-2
Journal: Comput Biol Chem
Published: 2022
Background
The emergence of COVID-19 caused by SARS-CoV-2 and its spread since 2019 represents the major public health problem worldwide nowadays, which has generated a high number of infections and deaths. The spike protein (S protein) is the most studied protein of SARS-CoV-2, and key to host-cell entry through ACE2 receptor. This protein presents a large pattern of glycosylations with important roles in immunity and infection mechanisms. Therefore, understanding key aspects of the molecular mechanisms of these structures, during drug recognition in SARS-CoV-2, may contribute to therapeutic alternatives. In this work, we explored the impact of glycosylations on the drug recognition on two domains of the S protein, the receptor-binding domain (RBD) and the N-terminal domain (NTD) through molecular dynamics simulations and computational biophysics analysis.
Results
The study investigates the combined impact of glycosylation and ligand interactions on the receptor-binding domain (RBD) and N-terminal domain (NTD) of SARS-CoV-2 and illustrates six systems created with varying conditions, including the presence or absence of glycosylations and ligands. The top row represents systems without glycosylations, serving as controls. The middle row depicts systems with the ligand TCMDC-124223 interacting with RBD, while the bottom row shows systems with the ligand TCMDC-133766 interacting with NTD. The figure clearly distinguishes between systems with and without glycosylations, providing a visual representation of the experimental setup (Figure 1).
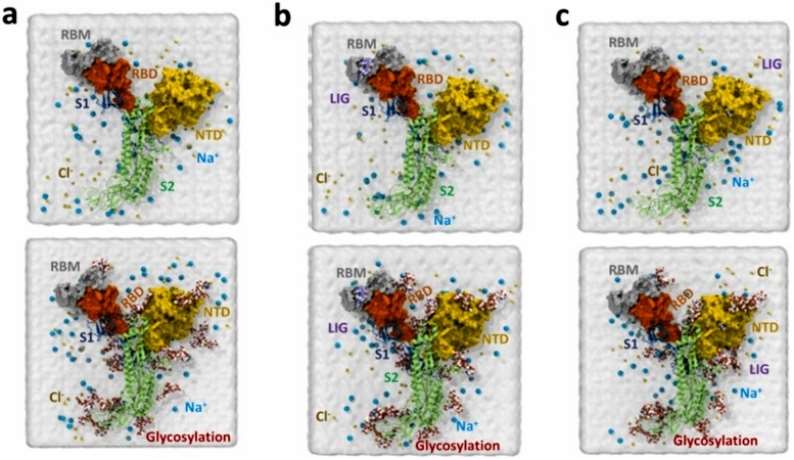 Figure 1
Figure 1
Our RMSD analysis suggests that both RBD and NTD have similar flexibility independent of glycosylation in the APO structure. However, the glycosylated protein presented a slightly higher RMSD, and especially in the RBD, a conformational change of ~2 Å after 250 ns was observed. In terms of the motifs of each region, they maintain a similar flexibility in both states, which can be distinguished in the heat map (Figure 2).
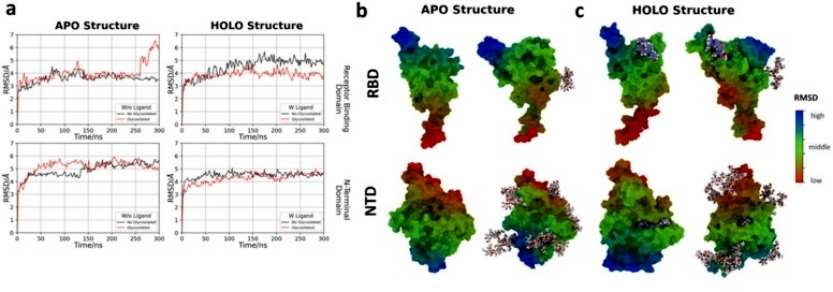 Figure 2
Figure 2
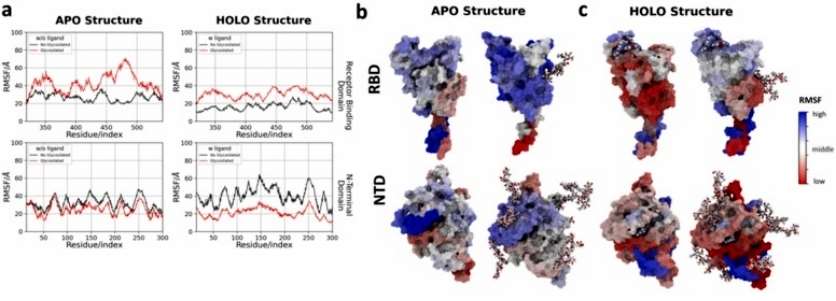
To evaluate changes in the rigid/flexibility of protein structure, RMSF analysis of the backbone was performed. The glycosylated and non-glycosylated NTD structures without ligand (Apo) did no presented differences about flexibility, with maximum RMSF values of ~50 Å. The author observe that the motifs in the NTD are much rigid only when they are close to a glycosylation. In contrast, the RBD region tends to be much more flexible across the domain, even with a single glycosylation (Figure 3).
The molecular dynamics of the RBD structures revealed that the ligand undergoes through different conformational states promoted by roto-translational phenomena in the RBM region in the absence of glycosylations. These changes are observed from early stages of molecular dynamics, followed by a perpendicular rotation at 29.7 ns and translation until 60 ns, finally the ligand remains at the opposite end of RBM until the end of the simulation. In contrast, glycosylation induces a sustained ligand interaction in loop/β structure formed by residues T470-F490, which promote less conformational changes and rotations until the end of simulation (Figure 4).
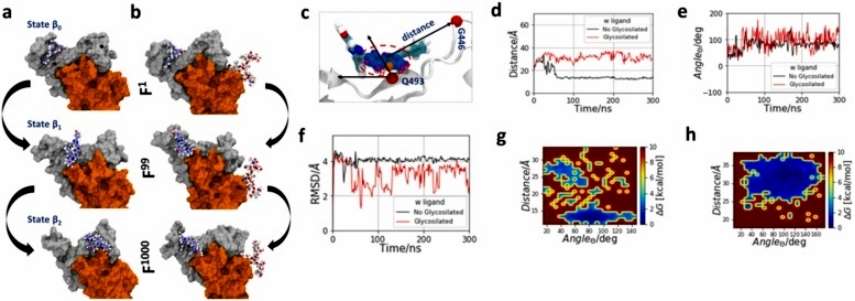 Figure 4
Figure 4
Conclusion
The study investigates the influence of glycosylations on drug recognition within two domains of the SARS-CoV-2 spike protein—the receptor-binding domain (RBD) and the N-terminal domain (NTD). Employing molecular dynamics simulations and computational biophysics analysis, the research reveals that glycosylations induce structural stability and alter the rigidity/flexibility of the S protein. These structural modifications are deemed crucial for its biological activity and the proper interaction of ligands in the RBD and NTD regions. Notably, the presence of glycosylation affects the roto-translation phenomenon in the ligand-RBD interaction, indicating a significant impact on metastable states in the RBD region. Furthermore, glycosylations in the NTD prompt an induced fit phenomenon, essential for ligand activity at the cryptic site. The findings emphasize the importance of glycosylation in the biophysical properties and drug recognition of the SARS-CoV-2 S protein, highlighting its significance in rational drug development and virtual screening efforts targeting the S protein.
Reference
Our products and services are for research use only.