
- Home
- PTMs Proteomics
- Glycoproteomics Analysis
- N-glycosylation profiling of Proteins
Protein glycosylation is one of the most common and complex post-translational modification types in organisms. In eukaryotic cells, approximately 50% of proteins undergo glycosylation. The two most common forms of glycosylation are N-linked glycosylation and O-linked glycosylation. N-linked glycosylation of proteins is of paramount importance as one of the key post-translational modifications. It plays a crucial role in complex multicellular or tissue formation, contributing to processes such as cell-cell adhesion, receptor-ligand interactions, immune responses, apoptosis, and various disease mechanisms. N-linked glycosylation sites on proteins exhibit a conserved amino acid sequence NX (S/T), where X represents any amino acid except proline. Common N-glycan types include High Mannose, Complex/Hybrid, Sialylation, Fucosylation, Xylosylation, and Glycan-phosphorylation (M6P).
Glycans play pivotal roles in various essential biological processes, including endocytosis, immune cell differentiation and activation, cancer development, and metastasis. In recent decades, mass spectrometry analysis has made significant strides, expanding its applications in glycan research and advancing the field. The evolution of soft ionization techniques has revolutionized the analysis of biomacromolecules, while the development of tandem mass spectrometry has provided a robust platform for glycan sequence determination and detailed structural analysis. The integration of mass spectrometry with orthogonal techniques for N-glycan analysis further compensates for the limitations of single mass spectrometry approaches.
In recent years, mass spectrometry-based N-glycosylation analysis has witnessed remarkable progress. Derivatization reactions and various labeling methods have found wide use in glycomics research. To categorize these methods based on whether derivatization or labeling is applied to the sample and whether the goal is quantitative analysis, glycomics quantitation approaches can be divided into labeled and label-free glycomics analysis methods.
Mass spectrometry-based N-glycoproteomics allows for comprehensive analysis at different molecular levels, including intact N-glycoproteins, intact N-glycopeptides, N-glycosylated peptide site occupancy, and N-linked glycans. The results obtained at different molecular levels complement each other, providing qualitative and quantitative insights into N-glycosylation. This approach aids in the study of site-specific N-glycoprotein structures and functions, serving as a valuable tool in disease diagnosis, prognosis, drug target discovery, and the development of highly targeted biopharmaceuticals.
| Analysis Type | Qualitative Information | Quantitative Information |
|---|---|---|
| N-Glycopeptide - Site-Specific and Structure-Specific N-Glycosylation Analysis | - Peptide backbone amino acid sequences - N-glycosylation sites - N-linked sugar monomer composition/sequence/linkage - Corresponding N-glycoproteins |
- Relative changes in site-specific and structure-specific N-glycosylation between experimental and control groups |
| N-Linked Sugar - Structure-Specific N-Glycosylation Analysis | - N-linked sugar monomer composition - Sequence - Linkage |
- Relative changes in structure-specific N-glycosylation between experimental and control groups |
| N-Glycosylated Peptide - Site-Specific N-Glycosylation Analysis | - Peptide backbone amino acid sequences - N-glycosylation sites |
- Relative changes in site-specific N-glycosylation between experimental and control groups |
| Intact N-Glycoprotein - Site-Specific and Structure-Specific N-Glycosylation Analysis | - Protein backbone amino acid sequences - N-glycosylation sites - N-linked sugar monomer composition/sequence/linkage |
- Relative changes in site-specific and structure-specific N-glycosylation between experimental and control groups |
The inherent complexity of glycan molecules and the low expression levels of many N-glycoproteins pose significant challenges for characterizing N-glycosylation structures. Detecting low-abundance N-glycosylated proteins or peptides often involves a substantial presence of unmodified protein peptides, which can easily mask the signals from low-abundance glycosylated protein peptides. To address this issue, lectin affinity enrichment is currently employed.
Lectin affinity is a widely used separation and enrichment method in glycoproteomics. Lectins are a class of sugar-binding proteins capable of specifically recognizing certain sugar or glycan structures and binding to them. They form reversible non-covalent complexes with sugar chains. After capturing glycoproteins or glycopeptides with lectins, these glycoproteins or glycopeptides are typically eluted using specific sugars that competitively bind to the lectin. Following enzymatic digestion, N-glycosylated peptide segments are enriched using lectins. Subsequently, N-glycanase (PNGase) is used to cleave the glycan chains attached to asparagine residues (Asn) in H2¹⁸O, resulting in an increase of 2.9890 Da in Asn molecular weight. Finally, the deglycosylated peptide segments are detected using high-precision LC-MS spectrometry. By comparing the changes in molecular weight after deglycosylation with their theoretical molecular weights and identifying the sequences of glycosylated peptide segments through database searches, the N-glycosylation sites of the protein are determined.
Following the determination of N-glycosylation sites, a label-free approach is employed for quantitative analysis.
Analysis of changes in the glycoproteome under different experimental conditions and signaling pathways.
Screening of candidate glycoprotein biomarkers.
Orbitrap Series (Thermo), Tims TOF Pro2 (Bruker)
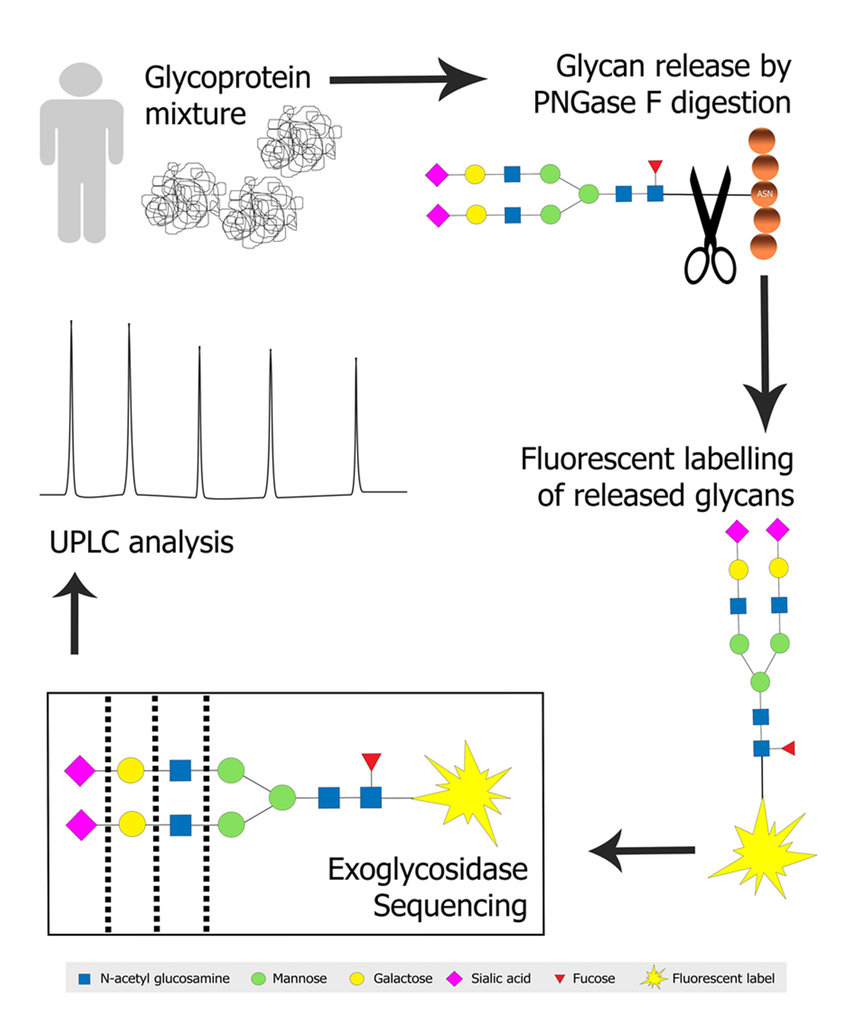 N-glycan profiling of a glycoprotein mixture. (Patricia Regan et al,. Medicines 2019)
N-glycan profiling of a glycoprotein mixture. (Patricia Regan et al,. Medicines 2019)
Identification and Analysis of N-Glycosylation Sites in Large-Scale Proteomes.
High-throughput and high-sensitivity N-Glycan profiling
For biopharmaceutical development and disease biomarker discovery etc.
| Sample Type | Sample Requirements |
|---|---|
| Plant Leaves | Wet weight ≥ 3g |
| Plant Roots, Stems, Bark, etc. | Wet weight ≥ 5g |
| Cell Samples | Cell count (wet weight) ≥ 5*107 |
| Tissue Samples (Human, Animal, Microbial) | Wet weight ≥ 50mg |
| Fluid Samples (Serum, Plasma) | Volume ≥ 200μl |
| Fluid Samples (Cerebrospinal Fluid) | Volume ≥ 500μl |
| Fluid Samples (Urine) | Volume ≥ 1000μl |
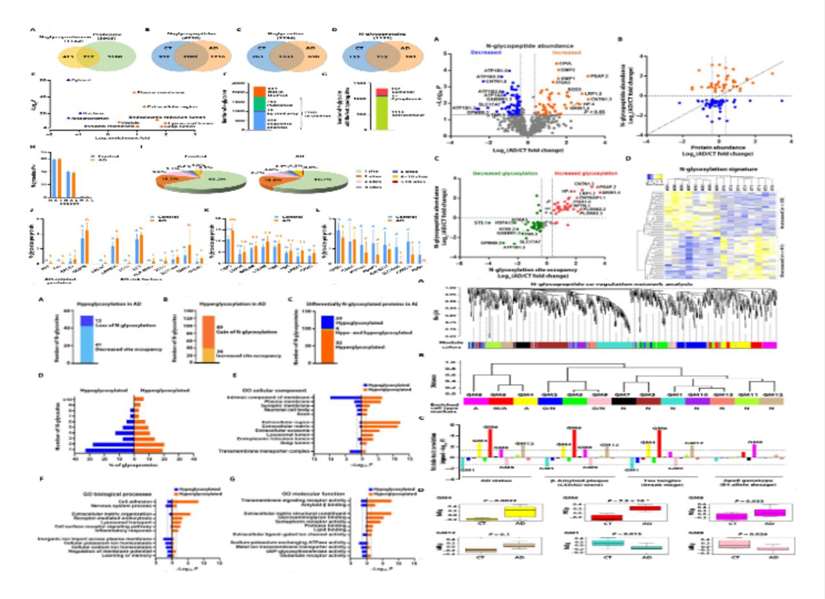
Integrative glycoproteomics reveals protein N-glycosylation aberrations and glycoproteomic network alterations in Alzheimer's disease
Journal: Sci Adv
Impact Factor: 14.957
Technical Method: Quantitative N-Glycoproteomics
Research Content
Glycosylation defects are a significant feature of many neurological disorders, yet our understanding of their systemic changes in Alzheimer's disease (AD) remains limited. This study describes a large-scale site-specific N-glycoprotein atlas analysis, employing high-resolution mass spectrometry-based quantitative N-glycoproteomics, to characterize a comprehensive panorama of N-glycoproteins and N-glycosylation sites in AD patient and control brain samples. The article identifies 1132 N-glycoproteins and 2294 N-glycosylation sites, integrating to construct a co-regulatory network of N-glycopeptides/glycoproteins. This provides novel insights at both the molecular and systemic levels for understanding and treating AD.
Results
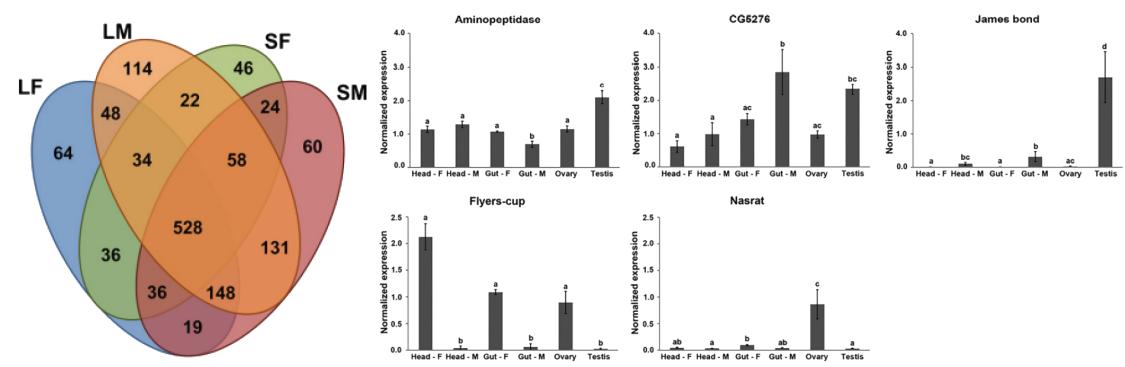
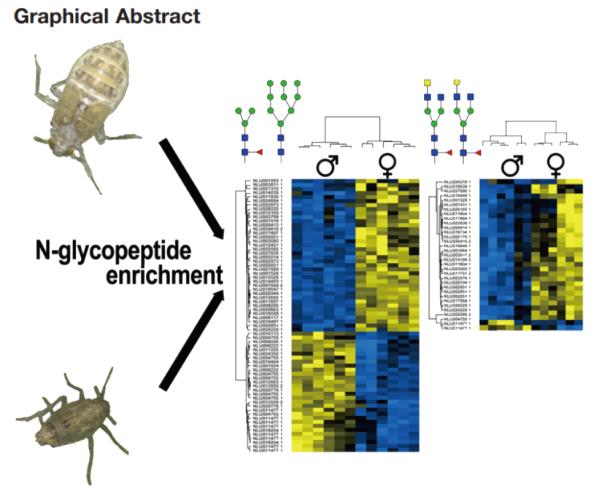
N-glycosylation Site Analysis Reveals Sex-related Differences in Protein N-glycosylation in the Rice Brown Planthopper (Nilaparvata lugens)
Journal: Mol Cell Proteomics
Impact Factor: 7.381
Technical Method: N-Glycoproteomics
Research Content
Glycosylation is a common protein modification that plays a crucial role in a wide range of biological processes. Gender differences in protein glycosylation have been observed in humans, nematodes, and schistosomes. This study also identified differences in protein glycosylation in the rice pest, brown planthopper. A comprehensive study of N-glycosylation sites in brown planthopper adults was conducted, followed by qualitative and quantitative comparisons at the glycopeptide level between genders. The analysis identified over 1300 N-glycosylation sites from more than 600 glycoproteins, revealing significant differences in N-glycosylation between genders. This provides new insights into the biology of the brown planthopper and offers information for future biocontrol strategies."
Approach
References
Our products and services are for research use only.