Proteomics, a dynamic field of study, is significantly influenced by post-translational modifications (PTMs). These modifications play a crucial role in finely tuning protein structure, function, stability, cellular localization, and enzymatic activity. Among these, citrullination, a specific PTM, involves the conversion of arginine residues into citrulline through an enzymatic reaction mediated by peptidylarginine deiminase (PAD) family members. While the precise details of this conversion mechanism during standard cellular activities remain incompletely understood, it has been observed to primarily occur in response to stress conditions. These conditions encompass a range of biological processes such as inflammation, autophagy, and elevated intracellular calcium levels, including apoptosis, necrosis, and oxidative stress.
Citrullination: Function and Mechanism
Biochemistry of Citrullination:
citrullination is a post-translational modification mediated by PAD enzymes, which facilitate the conversion of arginine residues in proteins to citrulline. This enzymatic reaction involves the hydrolysis of a water molecule and the release of ammonia. The substitution of the positively charged arginine with the neutral citrulline results in significant changes to the protein's structure and functionality. Given the crucial role of arginine in protein-protein interactions, DNA binding, and signal transduction, citrullination can profoundly influence these fundamental biological processes.
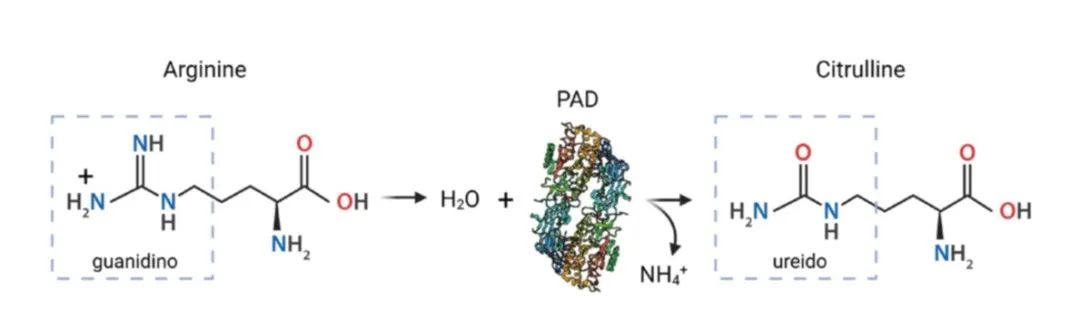 Figure 1: The Citrullination Process
Figure 1: The Citrullination Process
Role of Citrullination in Cellular Processes:
Citrullination is integral to numerous cellular processes, including the regulation of gene expression, cell division, apoptosis, and immune responses. This post-translational modification affects cellular function by altering protein-protein interactions and modifying structural stability. For example, citrullination can impact chromatin architecture, thereby influencing gene activation or repression. Additionally, citrullination plays a crucial role in the immune system, where it contributes to the generation of autoantigens and the mechanisms underlying immune evasion.
Context of Citrullination in Complex Diseases:
Recent research has highlighted the significant role of citrullination in the pathogenesis of complex diseases and its connection to the innate immune system (Table 1). In rheumatoid arthritis (RA), anti-citrullinated protein antibodies (ACPAs) can be detected years before the onset of clinical symptoms. These antibodies are utilized as diagnostic biomarkers in approximately 70% of RA cases and are associated with disease prognosis.
In oncology, elevated levels of PAD enzymes have been observed in various cancers, suggesting that citrullinated proteins may serve as valuable diagnostic markers. For instance, PAD4 has been detected in the blood of patients with breast, lung, colorectal, ovarian, and prostate cancers.
The link between citrullination and neurodegenerative diseases has also been investigated. In Alzheimer's disease, citrullinated β-amyloid proteins have been identified in the brain. Additionally, a meta-analysis of blood metabolites in dementia patients revealed significantly increased levels of citrulline. Structural analyses have pinpointed a potential citrullination site in the TDP-43 protein of patients with frontotemporal lobar degeneration. This underscores the need for novel diagnostic strategies targeting autoantibodies against citrullinated proteins, an area that remains ripe for exploration.
| Disease Type | Findings |
|---|---|
| Rheumatoid Arthritis | Anti-citrullinated protein antibodies are detected in 70% of cases, serving as a diagnostic biomarker. |
| Breast Cancer, Lung Cancer, Colorectal Cancer, Ovarian Cancer, Prostate Cancer | Presence of PAD4 (Peptidylarginine deiminase type 4) in blood samples. |
| Alzheimer's Disease | Citrullinated β-amyloid plaques identified in the brain. |
| Dementia | Increased levels of citrulline in blood metabolite analyses. |
| Frontotemporal Dementia | Potential citrullination sites identified in TDP-43 protein. |
Table 1: Role of Citrullination in Complex Diseases
Challenges and Advances in the Detection of Citrullination
The detection and study of citrullination, a low-abundance PTM, necessitate highly sensitive analytical techniques. While traditional methods such as enzyme-linked immunosorbent assay (ELISA) and Western blotting (WB) provide insights into the distribution of citrullinated proteins and PADs, they are limited by low throughput and semi-quantitative analysis. High-throughput and sensitive methods, such as mass spectrometry (MS) and protein microarrays, have emerged as powerful tools for the comprehensive analysis of citrullination. Each technique, however, presents distinct advantages and limitations regarding sample preparation and analytical specificity.
Mass Spectrometry in Citrullination Detection
MS is a highly effective analytical technique used for the direct identification and quantification of citrullinated proteins. The advantages of MS-based methodologies include exceptional sensitivity, specificity, and the capacity to analyze complex protein mixtures. The application of MS in citrullination research has significantly advanced our understanding of PTMs at a proteomic level, enhancing insights into their roles in disease pathology.
Technical Challenges and Methodological Considerations
A major challenge in the MS-based detection of citrullination is the accurate differentiation of citrullinated residues from similar modifications, such as the deamidation of glutamine or asparagine. Both modifications induce a mass shift of approximately 0.984 Da, which complicates the precise identification of citrullination. To address this challenge, advanced techniques such as LC-MS/MS combined with specific enrichment strategies have been developed. For instance, Fadzen et al. (2018) introduced a novel method involving anti-citrulline antibodies for the enrichment of citrullinated peptides, followed by high-resolution MS for accurate quantification.
Applications in Disease Research
The utility of MS in studying citrullination has been demonstrated in various diseases, including RA and multiple sclerosis. In RA, citrullinated peptides are known to be targets of autoantibodies, which are detectable years before the onset of clinical symptoms. For example, a study by Nielen et al. (2004) demonstrated the use of MS to identify and characterize citrullinated peptides in the serum of RA patients. This study provided significant insights into the early detection and autoimmune mechanisms involved in RA.
Recent Advances and Future Directions
Recent advances in MS technology, such as the development of Orbitrap and time-of-flight (TOF) analyzers, have significantly improved the resolution and accuracy of citrullination detection. Additionally, novel labeling techniques and bioinformatics tools have enhanced the quantitative analysis of citrullinated peptides. Future research is expected to focus on integrating MS with other omics technologies, such as transcriptomics and metabolomics, to provide a comprehensive understanding of citrullination's role in cellular processes and disease mechanisms.
Select Service
Protein Microarrays in Citrullination Detection
Protein microarrays facilitate the screening of citrullinated proteins through immobilized protein arrays. Direct methods utilize labeled PAD enzymes to screen fixed proteins, while indirect methods detect autoantibodies against citrullinated proteins. These approaches offer opportunities for disease detection, subtype classification, and the identification of novel therapeutic targets.
Sengenics' Protein Array Technology: Selecting an appropriate protein array is crucial for generating accurate data in citrullination research. Sengenics has distinguished itself in this field by offering a citrullination detection method that leverages its functional protein arrays. Utilizing proprietary KREX® technology, these arrays present full-length, correctly folded proteins. This precision contrasts with other arrays that may depend on denatured or misfolded proteins, which can lead to nonspecific antibody binding and unreliable results.
Conclusion and Future Perspectives
The pivotal role of citrullination in various diseases, particularly autoimmune conditions such as rheumatoid arthritis and certain cancers, is increasingly recognized. Understanding the mechanisms and functions of citrullination can lead to the development of novel diagnostic tools and therapeutic strategies. The potential of citrullinated proteins as biomarkers and therapeutic targets opens new avenues for research and clinical applications.
Despite significant advances in citrullination research, several challenges remain. The methods for identifying and quantifying citrullinated proteins require further optimization to enhance sensitivity and specificity. Moreover, a deeper understanding of the specific mechanisms of citrullination in various diseases is essential for the development of targeted therapies.
Future research should focus on the following areas:
Development of more efficient methods for detecting citrullinated proteins to enable early diagnosis and monitoring of diseases.
Investigation of the specific mechanisms of citrullination in different diseases to elucidate its role in disease progression.
Exploration of the therapeutic potential of citrullination as a target, including the development of targeted therapies such as CAR-T cell therapy.
Evaluation of the dynamic changes in citrullination under healthy and diseased conditions to understand its role in normal physiological processes.
By addressing these challenges, the field can progress toward the comprehensive understanding and application of citrullination in medical science.
References
- van Beers, J. J., Willemze, A., Stammen-Vogelzangs, J., Korevaar, D. A., & Rispens, T. (2010). Identification of citrullinated peptides in synovial fluid from rheumatoid arthritis patients: Implications for diagnosis and understanding of disease pathology. Arthritis Research & Therapy, 12(2), R102.
- Nielen, M. M., van Schaardenburg, D., Reesink, H. W., et al. (2004). Anti-citrullinated protein antibodies in patients with rheumatoid arthritis and their relation to disease severity. Journal of Rheumatology, 31(12), 2398-2404.
Our products and services are for research use only.