Cellular metabolism is a complex network that meets the bioenergetic and biosynthetic needs of the cell. As a crucial component of post-transcriptional epigenetics, RNA modifications can influence various biological processes and cellular phenotypes.
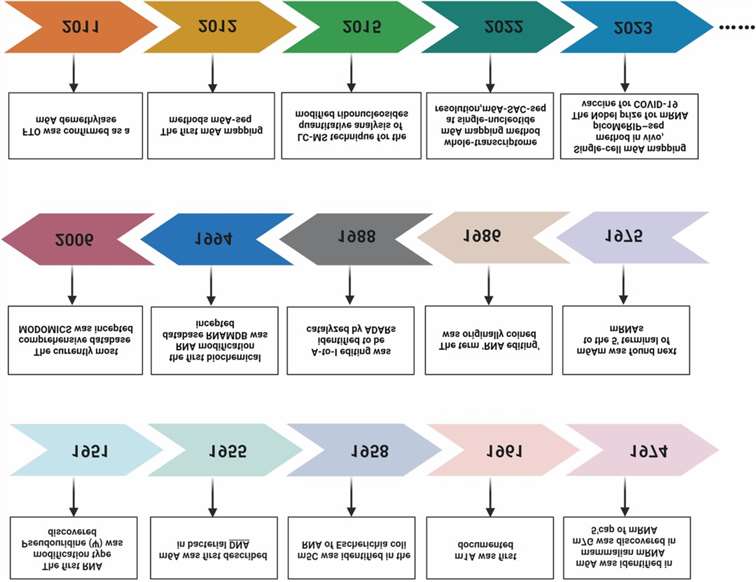 Figure 1. Milestones in the field of RNA modifications.
Figure 1. Milestones in the field of RNA modifications.
Main Types of RNA Modifications
RNA modifications can be classified into reversible and irreversible types. Reversible modifications typically involve smaller-scale changes to chemical side chains, ranging from simple methylation to the addition of large molecular entities. These plastic and reversible RNA modifications are widely involved in gene regulation and cellular states.
Irreversible RNA modifications mainly include RNA editing, splicing, and transcript content modifications (such as intron retention). In contrast to reversible modifications, these changes directly alter the sequence information, enhancing the plasticity and diversity of the transcriptome.
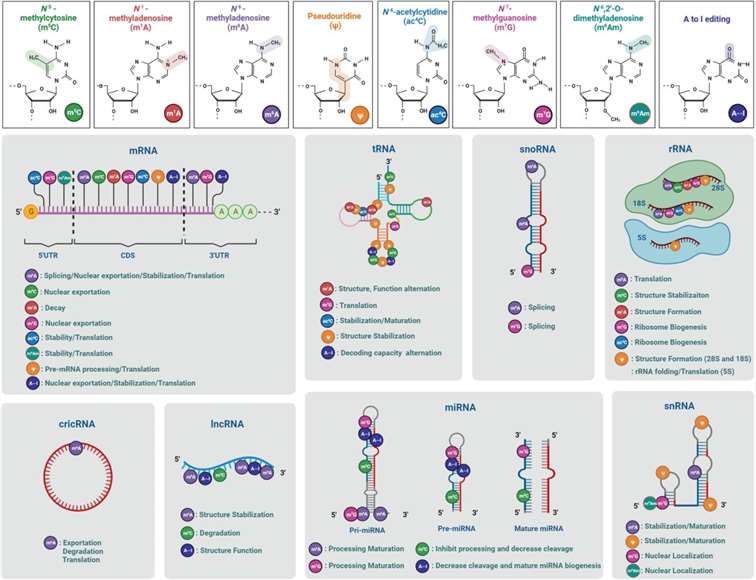 Figure 2. Chemical Structures, Distribution, and Molecular Functions of 8 Types of RNA Modifications
Figure 2. Chemical Structures, Distribution, and Molecular Functions of 8 Types of RNA Modifications
RNA Modifications and Cellular Metabolism
Cellular metabolism is a flexible network that enables cells to meet their bioenergetic and biosynthetic needs. In malignant tumor cells, metabolic reprogramming is closely associated with tumor initiation, progression, metastasis, and chemotherapy resistance. Beyond the well-studied cancer metabolism, the effects of metabolism are also widespread in various diseases, including diabetes, obesity, non-alcoholic fatty liver disease (NAFLD), and atherosclerosis. In these pathological processes, dysregulated RNA modification factors significantly contribute to metabolic changes by targeting metabolic enzymes, transport proteins, metabolism-related transcription factors, or pathways. This paper summarizes the effects of abnormal RNA modifications on glucose, lipid, amino acid, and mitochondrial metabolism and discusses how metabolism influences RNA modifications.
Glucose Metabolism
Glucose is the primary energy source for cells, and its metabolic pathways include aerobic oxidation, anaerobic glycolysis, the pentose phosphate pathway (PPP), glycogen synthesis, and gluconeogenesis. Glycolysis is a fundamental energy-producing process in living organisms, where glucose is broken down into pyruvate, releasing free energy in the form of ATP. Under normal conditions, after glycolysis in the cytoplasm, oxidative phosphorylation (OXPHOS) in mitochondria generates large amounts of ATP under aerobic conditions. However, in cancer cells, glycolysis is preferred over mitochondrial respiration, even when oxygen supply is sufficient, a phenomenon known as the Warburg effect. Notably, RNA modifications have been shown to play a key role in the glucose metabolism pathway by directly or indirectly regulating the expression of glycolysis-related genes (Figure 3).
Type 2 diabetes (T2D) is characterized by insulin resistance and hyperglycemia. The integrity of pancreatic β-cell function is crucial for maintaining blood glucose homeostasis. High glucose concentrations have been shown to reduce the levels of m6A in human and mouse islets. Importantly, the m6A modification plays a critical role in the biology of pancreatic β-cells. Abnormal glucose metabolism, characterized by enhanced glycolytic activity and lactate fermentation, is a fundamental component of metabolic reprogramming in tumors.
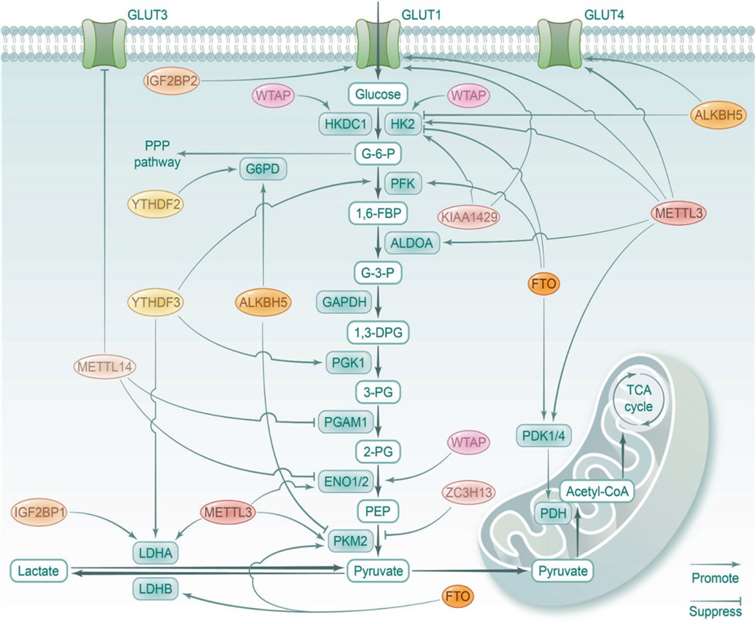 Figure 3. Role of RNA Modifications in Glucose Metabolism
Figure 3. Role of RNA Modifications in Glucose Metabolism
Lipid Metabolism
Lipids are essential components of biological membranes, the building blocks of biosynthesis, and crucial energy reserves. According to the integrated classification system, lipids are divided into fatty acids (FA), glycerolipids (GL), glycerophospholipids (GP), sphingolipids (SP), sterols (ST), prostaglandins (PR), glycolipids (SL), and polyketides (PK). Under conditions of high nutrient availability, fatty acids can be esterified and stored in lipid droplets, while under energy stress conditions, fatty acid oxidation (FAO), also known as β-oxidation, hydrolyzes to generate ATP. The synthesis of fatty acids is regulated by sterol regulatory element-binding protein 1c (SREBP1c). In response to growth factor stimulation, the precursor is processed into mature SREBP1c, which then translocates to the nucleus to enhance the transcription of target genes, including fatty acid synthase (FASN), acetyl-CoA carboxylase (ACC), stearoyl-CoA desaturase 1 (SCD1), and acly. Cholesterol is a precursor for the synthesis of fat-soluble vitamins and steroid hormones. Dysregulated lipid metabolism of GL, GP, and SP is associated with various pathological conditions, and RNA modifications are involved in multiple metabolic processes (Figure 4).
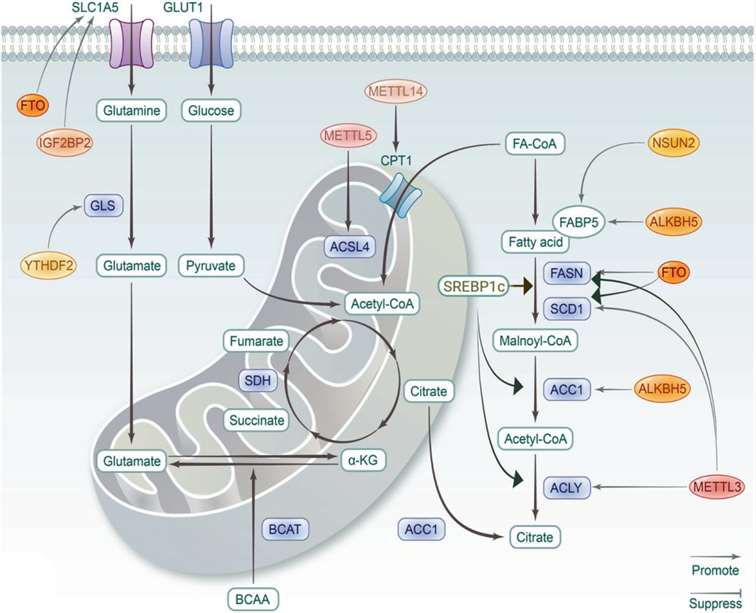 Figure 4. Role of RNA Modifications in Lipid and Amino Acid Metabolism
Figure 4. Role of RNA Modifications in Lipid and Amino Acid Metabolism
Mitochondrial Metabolism
As the metabolic center of the cell, mitochondria play an indispensable role in tumorigenesis. Aerobic glycolysis occupies a central position in the bioenergetic metabolism of tumors, but oxidative phosphorylation (OXPHOS) and mitochondrial-dependent energy supply are considered crucial for maintaining the stemness of certain tumor cells. In addition, providing substrates for anabolic metabolism, generating reactive oxygen species (ROS), and maintaining the regulatory cell death (RCD) signaling pathways are key to tumor progression. In both in vivo and in vitro experiments, FTO-mediated demethylation alleviates hepatic oxidative stress and mitochondrial fragmentation by targeting dynamin-related protein 1 (Drp1), exerting a protective effect in liver ischemia-reperfusion injury (HIRI) progression.
Amino Acid Metabolism
Amino acids, in addition to serving as substrates for protein or peptide synthesis, are also broken down through deamination or transamination to generate basic substrates for anabolic metabolism, such as α-keto acids used in the tricarboxylic acid cycle for energy release. Notably, glutamine has multiple biological functions beyond being a metabolic fuel or precursor for proteins. Glutamine catabolism is another significant feature of tumor metabolic reprogramming. Given that cancers often exhibit nutritional deficiencies in certain non-essential amino acids, targeting the supply of these amino acids has been proven to be an effective therapeutic intervention. Previous studies have confirmed the regulatory role of m6A modifications in glutamine metabolism. FTO-mediated m6A demethylation upregulates the expression of the glutamine transporter SLC1A5, and FTO inhibition, independent of HIF, suppressed the proliferation and viability of VHL-deficient clear cell renal cell carcinoma (ccRCC) cells.
m6A modifications play a critical role in inhibiting glutamine catabolism in colorectal cancer (CRC) cells and enhancing anti-tumor effects. In conclusion, as shown in Figure 5, numerous proteins and RNA modification-mediated metabolic processes are involved in the progression of various diseases. To translate these insights into practical prevention and treatment strategies, a thorough understanding of RNA modifications and metabolic disorders is essential.
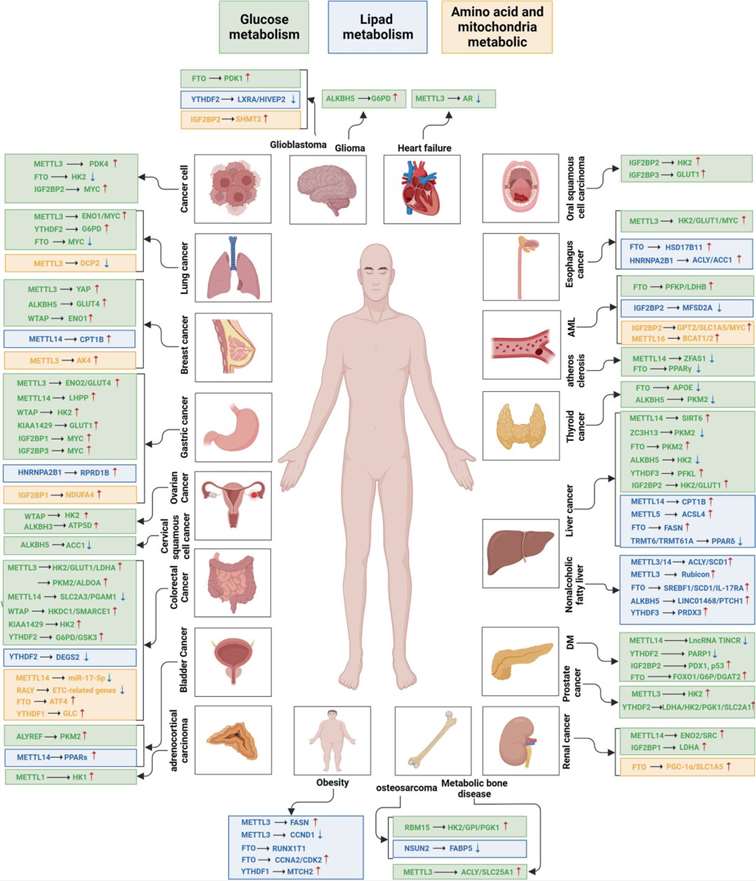 Figure 5. Epigenetic Regulation of Metabolism by RNA Modifications in Diseases
Figure 5. Epigenetic Regulation of Metabolism by RNA Modifications in Diseases
RNA Modifications and Immune Metabolism
In general, immune metabolism involves the distinction between activated and resting immune cells. The metabolism of activated immune cells is similar to that of malignant cells, characterized by minimal OXPHOS and the Warburg effect, while resting immune cells derive energy from fatty acid oxidation (FAO) and the Krebs cycle. Distinct metabolic patterns have been described for different immune cell subsets. This paper focuses on the contribution of RNA modifications to immune metabolism during various immune responses (Figure 6).
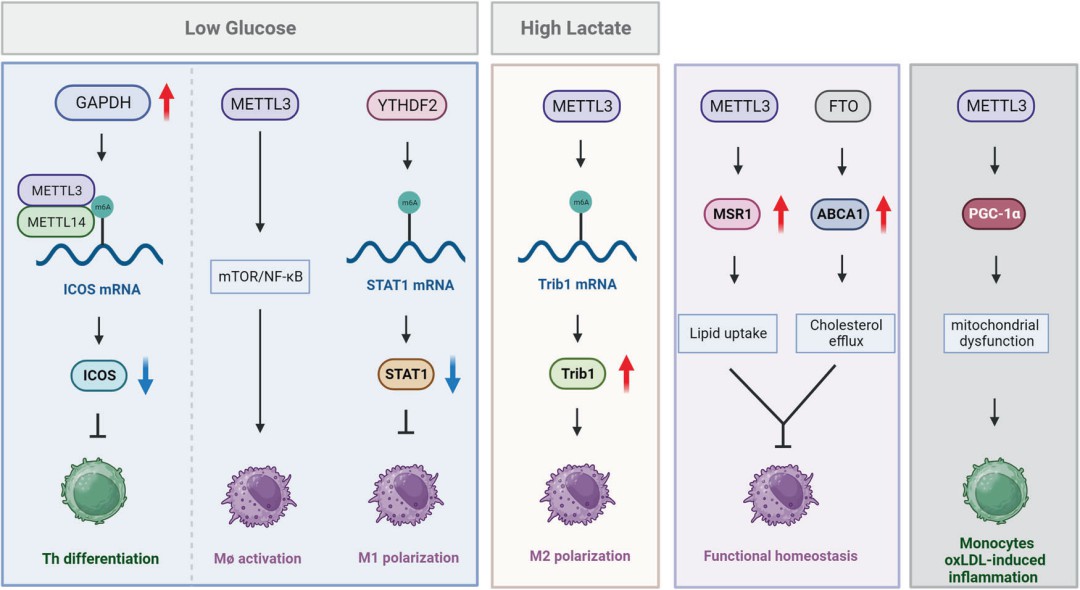 Figure 6. Impact of RNA Modifications on Immune Metabolism
Figure 6. Impact of RNA Modifications on Immune Metabolism
Anti-Tumor Immunity
The concept of the tumor immune microenvironment (TIME) emphasizes the interactions between immune cells, tumor cells, and other components of the immune system. These interactions, through the consumption of nutrients and the release of metabolic byproducts, influence immune responses. To sustain rapid proliferation, tumor cells require a substantial amount of glucose, glutamine, arginine, and other amino acids, which creates an unfavorable survival environment for immune cells. It is widely believed that tumor cell-induced glucose depletion contributes to the immunosuppressive TIME. Tumor cells often have an advantage over immune cells in glutamine consumption, as they overexpress the methionine transporter Slc43a2, thereby limiting methionine metabolism and impairing T cell anti-tumor function. However, whether RNA modifications are involved in the glutamine metabolism of immune cells remains to be explored. Several studies have confirmed that m6A methylation contributes to maintaining macrophage functional homeostasis by targeting the balance between lipid uptake and cholesterol efflux.
Anti-Viral Immunity
The pathogenesis of infectious diseases includes both immune system defects and immune evasion by pathogens. On one hand, specific RNA modifications on viral RNA, including m6A, m5C, ac4C, Ψ, and RNA editing, have been described, influencing the sensing and signaling of viral RNA. On the other hand, RNA modifications affect the function of immune cells and modulate the host's response to viral infections. The interferon pathway is the main target of m6A modification in regulating innate antiviral immunity. However, the relationship between RNA modifications and metabolic processes remains unclear.
Inflammation and Autoimmune Diseases
Inflammatory responses are mediated through coordinated gene expression programs, which include both acute and chronic forms of inflammation. These responses can be triggered by microbes, autoimmunity, allergies, metabolic disorders, and physical injury, leading to different types of inflammation. Recently, the regulatory role of RNA modifications in the expression of inflammatory and anti-inflammatory genes has been confirmed. Previous studies have shown that m6A modification is involved in the pathogenesis of autoimmune diseases. For example, METTL3 is significantly upregulated in rheumatoid arthritis (RA) patients and positively correlates with CRP and ESR, two common markers of RA disease activity. In systemic lupus erythematosus (SLE), reduced m5C levels and low expression of NSUN2 were observed in CD4+ T cells, and genes with upregulated hypermethylated m5C modifications were enriched in inflammatory pathways.
Select Service
Clinical Significance of RNA Modifications
RNA Modifications and the Treatment Response to Metabolic Therapies
For currently approved metabolic drugs, the main clinical challenge lies in drug resistance due to metabolic pathway reprogramming or compensatory metabolic pathways. Therefore, multi-pathway blockade or combination therapies may be more effective than monotherapy. Notably, several studies support the use of RNA modification-targeted therapies to improve chemoresistance to certain metabolic-targeted drugs. A typical example of this is the impact of m6A modification on the resistance of colorectal cancer (CRC) to 5-FU. In addition, inhibiting METTL14 has been shown to effectively resensitize GEM both in vitro and in vivo.
RNA Modifications and the Treatment Response to Immunotherapy
Studies have shown that m6A regulatory factors significantly affect the response to checkpoint blockade therapies. In vivo, the removal of FTO can decrease the expression of several key melanoma-promoting genes and enhance sensitivity to anti-PD-1 treatment. In addition to checkpoint blockade therapy, targeting m6A modifications has shown great potential in improving adoptive cell therapies. Encouraging progress has been made in regulating METTL3 and YTHDF2 in vitro to enhance NK cell proliferation and cytotoxicity, which may provide new strategies for NK cell-based immunotherapy. While there have been no reports on modulating m6A methylation in CAR T cells, considering the critical role of m6A regulatory factors in determining T cell function and fate, new therapeutic strategies are likely to emerge.
Development of RNA Modification-Targeted Drugs
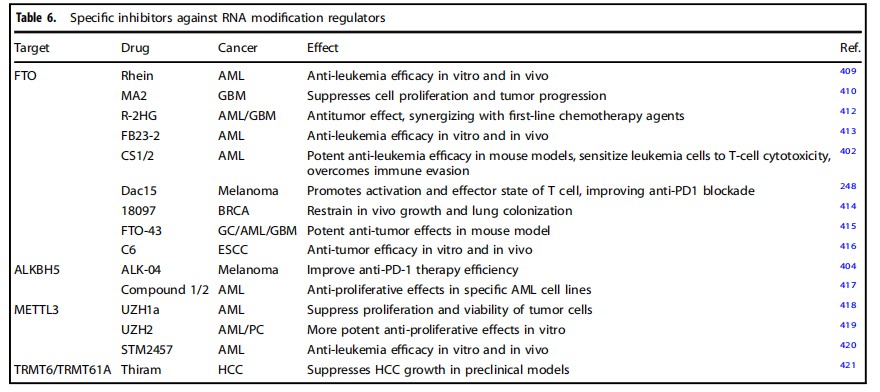
A range of specific inhibitors targeting dysregulated m6A regulators (which are often overexpressed in tumors) have shown anti-tumor effects both in vitro and in vivo (Table 6). FTO is considered one of the most promising targets. Over the past decade, a series of selective inhibitors, ranging from natural products to small molecule compounds, have been developed. The first natural inhibitor, emodin, demonstrated therapeutic activity in leukemia mouse models, while MA2 (methyl-chlorofenamic acid 2) was observed to inhibit glioblastoma progression.
Table: Specific Inhibitors Targeting RNA Modification Regulators
RNA Modifications in RNA-Based Therapies
Although RNA medicine has faced challenges such as efficacy and immunogenicity since its inception, the recent success of the anti-COVID-19 mRNA vaccines has reinvigorated the field, bringing renewed focus to RNA modifications. Chemical modifications of RNA can protect it from hydrolysis and nucleases, reducing off-target cytotoxicity. Once therapeutic RNA forms a double strand with the target sequence, modifications that lower the melting temperature can reduce the stability of the complex and improve target specificity by decreasing base pairing with non-target RNA. Additionally, RNA modifications can be used for RNA delivery and enhance the drug activity of RNA. Base modifications have been successfully applied to improve the performance of therapeutic RNA. For example, in COVID-19 vaccines, replacing uridine with the modified base 1-methylpseudouridine (N1-Me) effectively promotes translation and reduces off-target side effects and immunogenicity of therapeutic mRNA.
Combining Targeted RNA Modifications with Current Therapies
The combination of m6A regulator inhibitors with existing anti-tumor treatments has yielded some promising results. Targeting RNA modifications has shown synergistic effects in overcoming resistance and improving tumor-specific treatment. There is substantial evidence indicating that overexpression of METTL3 is widely involved in the resistance to various cancer therapies. Knockdown of METTL3 using short hairpin RNA can enhance the sensitivity of pancreatic cancer to chemotherapy agents. Targeting FTO has also provided new insights into improving chemotherapy resistance. The feasibility of applying m6A regulator inhibitors to enhance immunotherapy efficacy requires further investigation.
Reference
- Liu, WW., Zheng, SQ., Li, T. et al. RNA modifications in cellular metabolism: implications for metabolism-targeted therapy and immunotherapy. Sig Transduct Target Ther 9, 70 (2024). https://doi.org/10.1038/s41392-024-01777-5
Our products and services are for research use only.


.jpg)