Serotonin (5-hydroxytryptamine, 5-HT) is renowned for its role as a pivotal neurotransmitter in regulating mood, sleep, and appetite. Recent advancements, however, have identified its role in modulating cellular signaling through covalent protein modifications, a process termed serotonylation. This comprehensive analysis elucidates the scientific intricacies and recent developments in this domain:
Definition and Molecular Mechanism of Serotonylation
1. Chemical Nature
Serotonylation constitutes a post-translational modification (PTM), facilitated by transglutaminase (TGase). The enzyme catalyzes the formation of covalent bonds between the primary amine group (NH₂) of serotonin and the glutamine (Q) residues on target proteins, resulting in the formation of an irreversible isopeptide bond (ε-(γ-glutamyl)lysine isopeptide bond).
 Figure 1: Biochemical Pathways of Serotonylation (Jiang, et al., Journal of Cell Science, 2021)
Figure 1: Biochemical Pathways of Serotonylation (Jiang, et al., Journal of Cell Science, 2021)
2. Key Enzymes and Substrates
- TGase 2 (Tissue-type TGase): Predominantly catalyzes intracellular serotonylation, such as modification of small G proteins like RhoA and Rac1.
- FXIIIa (Coagulation Factor XIIIa): Facilitates serotonin modification of fibrinogen in platelets, promoting coagulation.
- Canonical Substrates: For instance, fibrinogen γ chain (FGG) in platelets undergoes serotonylation, enhancing platelet aggregation (Hummer et al., 2021).
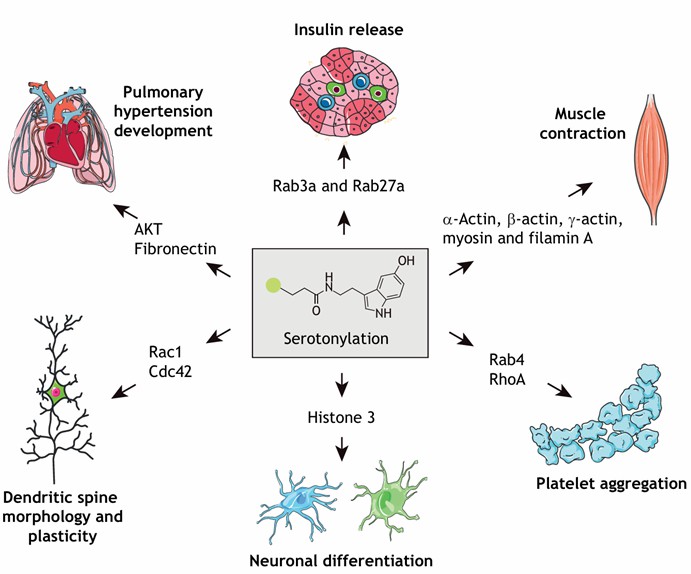 Figure 2: Substrates of Serotonylation and Their Associated Biological Functions (Jiang, et al., Journal of Cell Science, 2021)
Figure 2: Substrates of Serotonylation and Their Associated Biological Functions (Jiang, et al., Journal of Cell Science, 2021)
Biological Functions and Physiological Significance
1. Platelet Activation and Hemostasis
- Serotonin released from platelets modifies FGG, forming more stable fibrin clots, accelerating thrombogenesis (Nature Cardiovascular Research, 2022).
- Inhibition of serotonylation can mitigate pathological thrombotic risk, offering novel targets for anticoagulant therapies.
2. Neuroplasticity and Behavioral Regulation
- In hippocampal neurons, serotonylation of RhoA modulates synaptic plasticity and learning and memory processes (Cell Reports, 2020).
- Animal models illustrate that serotonylation deficiency can lead to anxiety-like behavior, implicating its relevance to mood disorders.
3. Modulation of Tumor Microenvironment
- Serotonin secreted by tumor cells modifies extracellular matrix proteins (such as fibronectin), enhancing cancer cell motility and angiogenesis (Science Signaling, 2023).
Disease Associations and Clinical Potential
1. Psychiatric Disorders
- Depression: Reduced serotonylation levels in platelets suggest a correlation with disrupted 5-HT signaling.
- Schizophrenia: Postmortem studies reveal abnormal TGase 2 activity in the prefrontal cortex, potentially affecting synaptic protein function.
2. Cardiovascular Diseases
- Elevated levels of serotonylated proteins detected in atherosclerotic plaques indicate their role in plaque stability.
3. Drug Development Directions
- TGase Inhibitors: Such as cystamine analogs, specifically block pathological serotonylation without impairing hemostatic function.
- Targeted Delivery Systems: Nanoparticles deliver inhibitors to thrombotic or tumor sites, reducing systemic side effects.
Detection Methods for Serotonylation Modifications
The detection of serotonylation modifications primarily involves immunoassays and mass spectrometry, or a synergistic application of both methodologies.
Site-Specific Antibody Detection
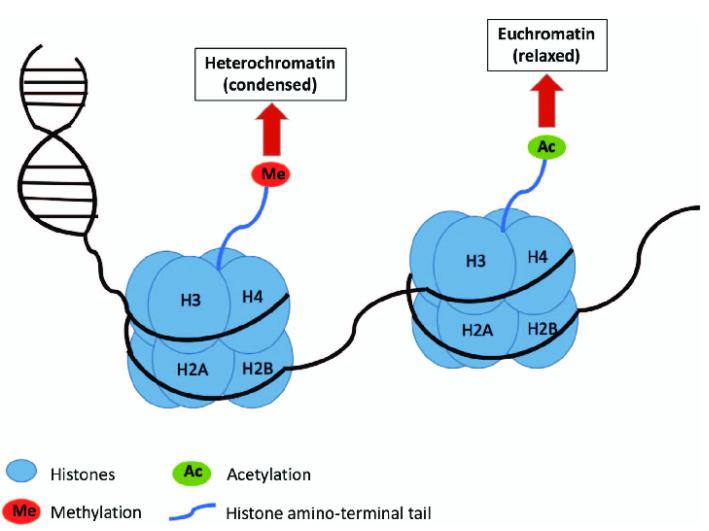
The modification level of target proteins is assessed through site-specific antibodies. For instance, a seminal study published in Nature in 2019 elucidated the role of histone H3 serotonylation in gene expression regulation. This investigation employed an H3Q5ser site-specific antibody to detect the serotonylation status of H3. The proteins precipitated through immunoprecipitation were subsequently identified using liquid chromatography-tandem mass spectrometry (LC-MS/MS), thereby confirming the presence of serotonylation at the H3Q5 position (refer to Figure 3).
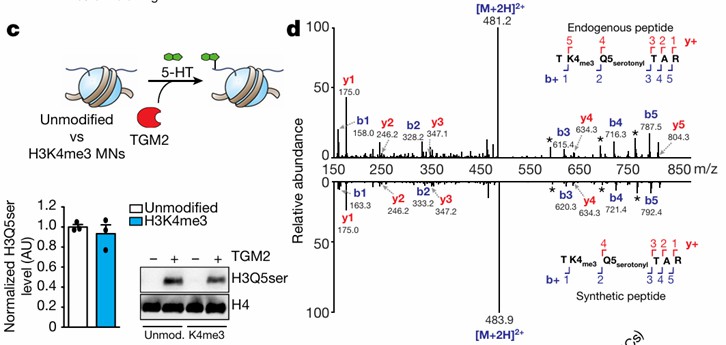 Figure 3: Detection of Histone Serotonylation Modifications (Farrelly, et al., Nature, 2019)
Figure 3: Detection of Histone Serotonylation Modifications (Farrelly, et al., Nature, 2019)
Chemical Proteomics for Enrichment and High-Throughput Detection of Serotonylation
The application of chemical proteomics involves the enrichment of serotonylated proteins for high-throughput analysis via mass spectrometry. In 2014, Ching-Yao Lin and colleagues developed an innovative approach utilizing an alkyne-containing serotonin derivative, 5-PT, as a probe. This probe is chemically linked via click chemistry to a biotin-containing tag. Streptavidin is subsequently employed to enrich the target proteins, which are then tagged, cleaved, and detected using mass spectrometry (refer to Figure 4) [3]. This method enables the comprehensive profiling of serotonylated proteins within tissue or cell samples, providing precise site-specific and quantitative information. Such advancements bolster the investigation of candidate molecular mechanisms.
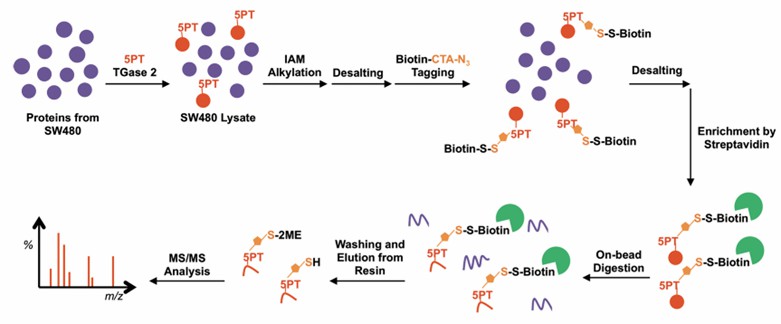 Figure 4: Workflow for Detection of Serotonylation Modifications via Chemical Proteomics
Figure 4: Workflow for Detection of Serotonylation Modifications via Chemical Proteomics
(Ching-Yao Lin, et al., Journal of Proteome Research, 2015)
Animal Models
- TGM2 knockout mice exhibit learning memory defects and coagulation abnormalities, validating serotonylation's multisystem functions.
Research Cases in Serotonylation Study
Histone Serotonylation Regulates Ependymoma Tumorigenesis
In examining the investigative paradigms of serotonylation, several high-impact publications offer illuminating insights. A key study is titled "Histone Serotonylation Regulates Ependymoma Tumorigenesis," published in Nature, featuring an impressive impact factor of 50.5. This research delves into the role of bidirectional communication between tumors and neurons within the malignant tumor microenvironment, with a particular focus on the dynamics of epigenomic dysregulation and exogenous neuronal signaling pathways in ependymoma (EPN) progression.
Initially, the study identifies serotonergic neurons as regulators of EPN tumorigenesis. Building on pre-existing knowledge, the authors investigate the influence of histone serotonylation modification (H3Q5ser) in EPN development. Their research validates that neuronal regulation of EPN is mediated via H3Q5ser by experimentally inhibiting serotonylation.
Subsequently, through high-throughput screening methods, such as chromatin immunoprecipitation sequencing (ChIP-seq), the downstream molecular pathways governed by H3Q5ser were explored. It was discovered that the transcription factor ETV5 promotes EPN progression by enhancing repressive chromatin states.
Finally, the study reveals that ETV5 regulates the expression of neuropeptide Y (NPY), which in turn inhibits tumor progression through synaptic remodeling mechanisms. The comprehensive findings of this study highlight histone serotonylation as a critical driver of EPN tumorigenesis and elucidate the intricate interplay between neuronal signaling, neuroepigenomics, and developmental programs in propelling the malignancy of brain cancer.
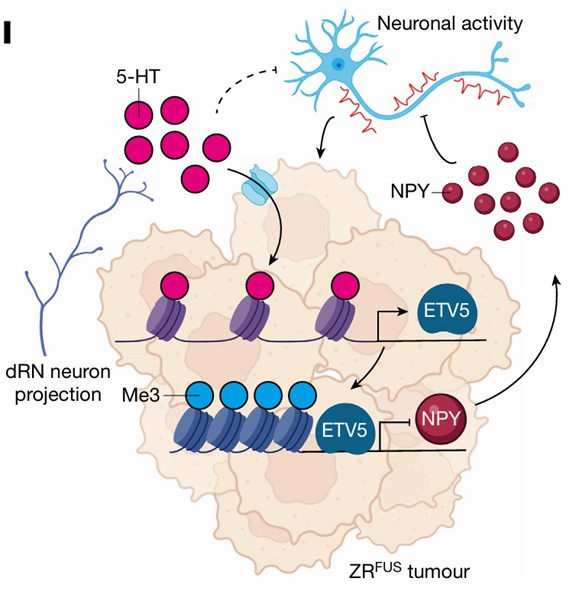 Figure 5: Schematic Model of Neuronal Serotonin Regulation in Tumor Progression (Chen, et al., Nature, 2024)
Figure 5: Schematic Model of Neuronal Serotonin Regulation in Tumor Progression (Chen, et al., Nature, 2024)
A GAPDH Serotonylation System Couples CD8+ T Cell Glycolytic Metabolism to Antitumor Immunity
Researchers have elucidated a novel post-translational modification mechanism involving the serotonylation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which enhances the glycolytic metabolism and antitumor immune activity of CD8+ T cells. Initially, it was observed that serotonin directly stimulates the proliferation of CD8+ T cells under αCD3/αCD28 stimulation. Through chemical proteomics, GAPDH was identified as a substrate of serotonin modification. This modification is correlated with increased glycolytic metabolism in activated CD8+ T cells.
To identify the serotonylation sites on GAPDH, the authors conducted liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, revealing that glutamine residues at positions 46, 76, 183, 202, and 262 on synthetic murine GAPDH peptides can undergo serotonylation. The critical role of serotonylation at the Q262 site was experimentally verified.
Subsequent analyses uncovered the role of the serotonin modification system, mediated by tissue transglutaminase 2 (TGM2), in CD8+ T cell activation and antitumor immunity, encompassing serotonin synthesis, transport, and degradation. The therapeutic potential of serotonin-modified CD8+ T cells in tumor immunotherapy was evaluated, specifically in the context of chimeric antigen receptor T (CAR-T) cell antitumor efficacy. It was found that overexpression of tryptophan hydroxylase 1 (TPH1) in CAR-T cells, which augments serotonin production, enhances antitumor efficacy.
In this study, Biopu Technologies provided the LC-MS/MS mass spectrometry analysis, which was instrumental in identifying serotonylated proteins within CD8+ T cells and determining the specific serotonylation sites on GAPDH.
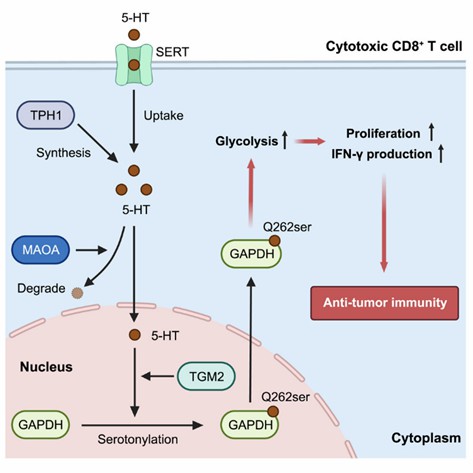 Figure 6: Model of GAPDH Serotonylation in Regulating CD8+ T Cell Antitumor Immunity
Figure 6: Model of GAPDH Serotonylation in Regulating CD8+ T Cell Antitumor Immunity
( Wang, et al., Molecular Cell, 2024)
Serotonylation in Tumor-Associated Fibroblasts Contributes to the Tumor-Promoting Roles of Serotonin in Colorectal Cancer
Previous investigations have highlighted the multifaceted roles of serotonin in colorectal cancer (CRC) biology. While serotonin predominantly exerts its effects through interaction with various serotonin receptors, receptor-independent mechanisms, such as post-translational modification by serotonin, remain inadequately understood in the context of CRC progression. This study demonstrates that silencing the gene encoding the serotonin-synthesizing enzyme tryptophan hydroxylase 1 (Tph1) or employing selective Tph1 inhibitors can significantly attenuate tumor growth in colorectal cancer.
Interestingly, although intrinsic serotonin synthesis occurs within CRC, the action of circulating serotonin mediates its pro-tumorigenic role. Functional blockade of serotonin receptors indicated that serotonin's oncogenic effects in CRC are mediated through receptor-independent pathways. Conversely, serotonylation of histone H3 at Q5 (H3Q5ser) was observed in colorectal cancer cells and cancer-associated fibroblasts (CAFs). This modification induces the phenotypic switch of CAFs towards an inflammatory-like (iCAF) subtype, which further enhances the proliferation, invasiveness, and macrophage polarization in CRC cells.
Reducing levels of H3Q5ser through knockdown of the serotonin transporter SLC22A3 or inhibition of transglutaminase 2 (TGM2) effectively mitigated the tumor-promoting phenotype of CAFs in CRC. Collectively, this research elucidates a serotonin-dependent mechanism in CRC progression and offers insights into potential therapeutic strategies targeting the serotonergic pathways in colorectal cancer treatment.
Future Challenges and Perspectives
1. Discovery of Unknown Substrates: Single-cell proteomics may reveal tissue-specific modification networks.
2. Dynamic Regulation Mechanisms: Investigate spatiotemporal variation of serotonylation under stress, inflammation, and other conditions.
3. Clinical Translation: Explore intervention strategies targeting neurodegenerative disorders such as Alzheimer's disease.
The discovery of serotonylation transcends the traditional notion of serotonin solely as a "free signaling molecule," providing an innovative perspective on the neuro-immune-metabolic crosstalk. As chemical biology and precision medicine converge, this field holds promise for revolutionary diagnostic and therapeutic methodologies.
Conclusion
Serotonylation, a recently characterized post-translational modification, represents one of the primary mechanisms through which serotonin exerts its biological functions. This modification has been widely implicated in the pathophysiological mechanisms of cancer, neurological disorders, and metabolic diseases. Target proteins for serotonylation include both histone and non-histone proteins. Notably, histone serotonylation primarily occurs at the H3Q5 site, where it can initiate the transcription of a downstream cascade of genes. In non-histone proteins, serotonylation alters substrate enzyme activity, cellular localization, protein-protein interactions, and protein conformation, thereby influencing the associated downstream phenotypes.
Current research on serotonylation is predominantly focused on identifying additional modification substrates and elucidating their regulatory mechanisms. Additionally, efforts are underway to develop specific therapeutics that target the serotonylation system to modulate its effects.
References
- Jiang SH, Wang YH, Hu LP, et al. The physiology, pathology and potential therapeutic application of serotonylation. J Cell Sci. 2021;134(11):jcs257337.DOI: 10.1242/jcs.257337
- Farrelly LA, Thompson RE, Zhao S, et al. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature. 2019;567(7749):535-539. https://doi.org/10.1038/s41586-019-1024-7
- Lin JC, Chou CC, Tu Z, et al. Characterization of protein serotonylation via bioorthogonal labeling and enrichment. J Proteome Res. 2014;13(8):3523-3529. DOI: 10.1021/pr5003438
- Chen HC, He P, McDonald M, et al. Histone serotonylation regulates ependymoma tumorigenesis. Nature. 2024;632(8026):903-910. https://doi.org/10.1038/s41586-024-07751-z
- Wang X, Fu SQ, Yuan X, et al. A GAPDH serotonylation system couples CD8+ T cell glycolytic metabolism to antitumor immunity. Mol Cell. 2024;84(4):760-775.e7. DOI: 10.1016/j.molcel.2023.12.015
- Ling T, Dai Z, Wang H, et al. Serotonylation in tumor-associated fibroblasts contributes to the tumor-promoting roles of serotonin in colorectal cancer. Cancer Lett. 2024;600:217150. DOI: 10.1016/j.canlet.2024.217150
Our products and services are for research use only.


.jpg)