The intricate coupling between metabolism and protein post-translational modifications (PTMs) has become a fundamental aspect of cellular regulation. Recent studies have shown that protein modifications can be derived from various metabolites, and their regulation is closely linked to the cellular metabolic state.
Modified proteins not only influence the associated metabolic pathways but also affect other signaling cascades involved in physiological processes and disease mechanisms. The recently discovered role of nuclear-localized metabolic enzymes in regulating histone modifications further underscores the importance of cell-type specific metabolic states.
Currently, protein post-translational modifications are a major focus of research. This article discusses recently discovered mechanisms that link metabolism, protein modifications, and protein function, as well as the contributions of various metabolic sources to protein modifications. It also explores how the emerging coupling between metabolism and PTMs influences physiological processes and disease mechanisms.
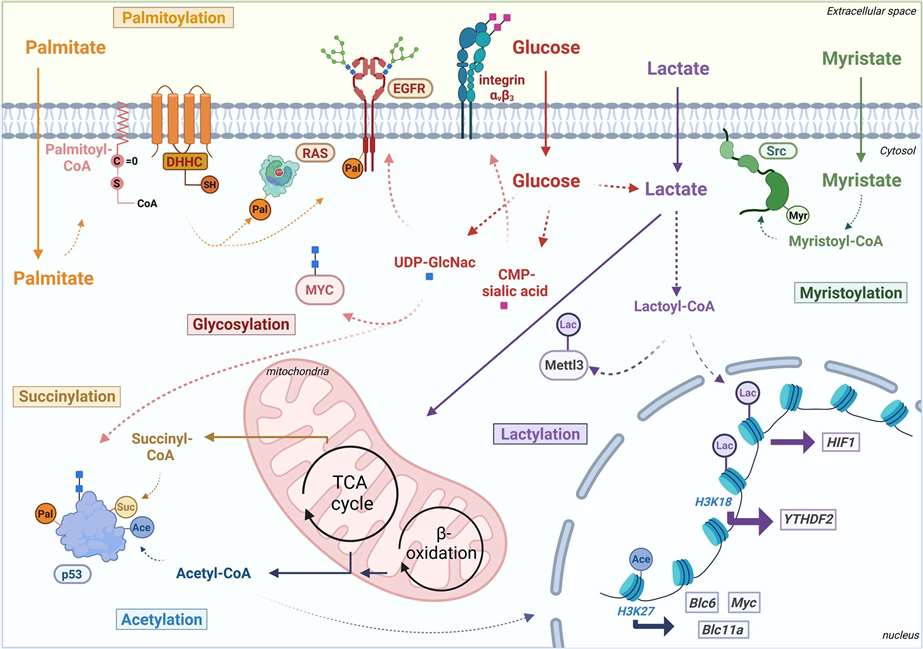 Figure 1 Schematic overview of metabolite-derived modifications palmitoylation (Pal), succinylation (Suc), glycosylation
Figure 1 Schematic overview of metabolite-derived modifications palmitoylation (Pal), succinylation (Suc), glycosylation
Select Service
Biochemical Diversity of Metabolite-Derived PTMs
Protein acylation is an early-identified form of protein modification, with new variants and functions continually being discovered. N-acylation primarily occurs through nucleophilic attack on activated acyl groups via the ε-NH2 of lysine or the α-NH2 at the N-terminus. The acyl groups involved are diverse, including acetyl, succinyl, and others. The abundance of acyl-CoA species varies significantly across different biological states and cellular compartments, presenting a challenge in distinguishing the functional roles of structurally similar protein acylations. Additionally, lysine benzoylation and isonicotinamidation are derived from drugs or food preservatives. N-acylation can also occur from O-acylated phosphates, such as in lysine 3-phosphoglyceroylation and lysine carbamoylation. Both short- and long-chain acyl groups can be attached to cysteine and serine residues, with cysteine S-acetylation potentially being quite prevalent.
Studies have shown that, in addition to lysine, serine, and cysteine, highly reactive metabolites can also modify other amino acid residues. For example, biochemical modifications of arginine, histidine, and tryptophan have been reported. The reactive carbonyl groups generated during glycolysis can react with arginine or lysine residues to form stable adducts. Peroxidation of polyunsaturated fatty acids (PUFAs) can produce 4-HNE, which modifies lysine, cysteine, and tryptophan residues. Recent examples of PTMs include the carbamoylation of lysine on ubiquitin by carbon dioxide, as well as formaldehyde modification of cysteine residues in the SAM synthase MAT1A.
In some instances, PTMs or amino acid residues containing PTMs can undergo further enzymatic or non-enzymatic modifications, resulting in functionally distinct modifications. For example, the thiol group in the reduced form of lipoic acid can react with CoA derivatives to produce inactive nitrosylated or glutaroylated acyl arms. Nε-monomethylated lysine can be further Nε-acetylated; in histones, acetylation of lysine marks transcription initiation sites. Cysteine residues can also undergo oxidation conjugation with various metabolites, and the relationship between this modification and metabolic regulation will be discussed in subsequent sections.
Select Service
Proximal PTMs Driven by Reactive Metabolites
Non-enzymatic PTMs are directly linked to metabolism, with fluctuations in metabolite levels leading to changes in modifications. Mitochondrial protein acylation primarily occurs through non-enzymatic processes, although exceptions exist. The high concentration of acyl-CoA in mitochondria results in the acylation of various proteins. An increase in mitochondrial pH promotes non-enzymatic acylation reactions. The level of acyl-CoA is directly coupled with protein function, such as the modification of GCDH by glutamyl-CoA, which influences lysine catabolism. Acylation of α-KGDH activates the complex, enhancing ATP production. The reduction of malonylation in ACC1 affects malonyl-CoA production. The regulation of protein function by acyl-CoA levels is modulated through acylation. In the mitochondria of mouse liver, proteins with CoA-binding pockets are more susceptible to acylation. Enzyme acylation, catalyzing the conversion of CoA or acyl-CoA, is part of a feedback loop that regulates physiological processes.
Acyl-phosphates influence protein function through covalent modification. For example, lysine residues within the substrate-binding domains of glycolytic enzymes are targets for modification by 1,3-bisphosphoglycerate, inhibiting glycolytic activity. Intermediate products of the urea cycle, such as carbamoyl phosphate, modify OTC, activating enzyme activity.
Although many PTMs are non-enzymatic, they often exhibit site specificity. For instance, modifications by (methyl)glyoxal, produced during glycolysis, and 4-HNE, generated through lipid peroxidation, are not random. Tyrosine residues are present at the +1 or +2 position of arginine, facilitating the generation of stable hydrogen-imidazolone isomers from (methyl)glyoxal derivatives. An example of selective 4-HNE modification is seen in human serum albumin.
Understanding the physiological significance of non-enzymatic protein modifications requires moving beyond methods that induce modifications via exogenous reactive metabolites. Gene interference can reveal underlying mechanisms, as locally generated reactive metabolites may rapidly react with nearby proteins. The specificity of non-enzymatic modifications may be driven by protein-protein interactions, with co-localized proteins often being part of the same "metabolome."
Metabolite Detection through Non-Enzymatic Protein Modifications
Changes in biological conditions affect the production and accumulation of metabolites, thereby altering the post-translational modifications (PTMs) derived from these metabolites. Non-enzymatic modifications primarily arise from the redox reactions or nucleophilic attacks involving acyl-CoA. Mitochondrial protein acylation levels are closely linked to acyl-CoA metabolism. For example, inhibiting complex I results in increased acetyl-CoA levels, which upregulate the inhibitory acetylation of SOD2 and suppress the growth of G3MB cells. Organic acidemia is associated with increased lysine acylation on proteins. In a propionic acidemia mouse model, insufficient propionyl-CoA carboxylase activity promotes histone H3 propionylation, leading to heart dysfunction.
In methylmalonic acidemia, lysine methylmalonylation causes mitochondrial enzyme inactivation. Symptoms can be alleviated by removing excess mitochondrial non-enzymatic acylation through sirtuin enzymes. Fatty acyl groups are post-translationally attached to many key metabolic enzymes. Conditions that promote fatty acylation modifications can gradually inactivate these enzymes, providing feedback to metabolic pathways. For instance, in activated CD8+ T cells, increased levels of glutarate lead to extensive glutamylation of PDH's acyl arms, regulating CD8+ T cell differentiation and enhancing cytotoxicity within tumors.
Metabolic alterations can lead to various oxidative modifications on cysteine residues. For example, elevated levels of homocysteine are linked to insulin resistance, which can be attributed to cysteine homocysteinylation at Cys825 of the insulin receptor. These examples highlight that non-enzymatic protein modifications serve as crucial mechanisms through which organisms respond to and adapt to metabolic and environmental changes.
Metabolic State and Enzymatic Protein Modification Kinetics
Research indicates that protein modifications are closely linked to the metabolic state under specific cell types and biological conditions. The levels of these modifications are influenced by the availability of enzymes, their selectivity, and cellular metabolites. For instance, the N-terminal acetylation catalyzed by N-terminal acetyltransferases is regulated by acetyl-CoA levels, affecting protein stability. The mechanisms maintaining essential protein modifications exhibit specificity across different species or cell types. Additionally, metabolite availability can influence sensitivity to HAT inhibitors, potentially contributing to cancer resistance. In an Alzheimer's disease mouse model, glycolytic reprogramming results in increased lactylation of histone lysines, exacerbating microglial dysfunction.
Protein fatty acylation levels correlate with the pool of free fatty acids. For example, when mammalian cells are cultured under glucose starvation, the levels of free fatty acids increase, concomitant with elevated cysteine acylation of proteins. In C. elegans, lipid metabolism is characterized by the production of substantial amounts of monomethyl branched-chain long-chain fatty acids, which serve as the primary source for protein fatty acylation. The specificity of protein fatty acylation may be influenced by different cellular compartments. The metabolic labeling of long-chain alkynyl fatty acids and click chemistry have become widely used methods for detecting and enriching fatty acylated proteins.
Enzymatic protein modifications such as hydroxylation, glycosylation, and glutaminylation are also linked to metabolism. For example, elevated polyamine levels in CD4+ T cells promote the hydroxylation of initiation factor eIF5A, maintaining the stability of T-helper cell lineages. In the brain, N-glycosylation derived from glucosamine is dependent on de novo glycogenolysis. Additionally, O-GlcNAcylation of proteins exhibits a circadian rhythm, regulating daily physiological cycles. In M2-like tumor-associated macrophages, increased O-GlcNAcylation correlates with enhanced metastasis and drug resistance. In adipocytes, high glutamine levels promote protein O-GlcNAcylation. The close relationship between O-GlcNAcylation and metabolism has been a subject of study. The accumulation of 5-hydroxytryptamine is essential for GAPDH-dependent CD8+ T cell activation and anti-tumor immunity.
Enzymatic protein modifications are also linked to dietary-induced metabolic changes. For instance, a ketogenic diet increases the production of β-hydroxybutyrate in the hippocampus. In a cocaine relapse mouse model, β-hydroxybutyrylation of protein lysines inhibits CAMKIIα activity and reduces the reinstatement of cocaine-seeking behavior. Dietary fibers metabolized by the microbiota serve as important carbon sources for the acetylation of histone H4 in epithelial cells. Diet-dependent free amino acids also modify protein lysine residues. For example, in mice fed a diet rich in phenylalanine, phenylalanine modification of the insulin receptor β results in impaired insulin signaling. ARS-catalyzed protein acylation is a newly discovered modification that may be more widespread than previously recognized.
Recent studies emphasize the complex interactions between the activation of metabolic pathways and associated protein modifications, which are often cell-type-specific and may have profound physiological impacts. Metabolites can also regulate protein modifications by modulating the enzymes associated with these modifications. For example, PRDX6 possesses sulfhydrolase activity and functions as a protein cysteine depalmitoylase, with its activity being dependent on glutathione. Short-chain fatty acids (SCFAs) inhibit histone deacetylases, inducing a high acetylation response, with SCFA-derived acetyl-CoA promoting acetylation via the p300-dependent acetyltransferase.
Nuclear-Localized Metabolic Enzymes and Histone Acetylation
The acetylation and deacetylation of histones regulate chromatin accessibility and gene expression, significantly impacting physiological processes. The metabolic state is closely linked to histone acetylation patterns, with particular attention given to the sources and regulatory mechanisms of acyl-CoA. Nuclear acyl-CoA generating enzymes play a crucial role in histone acetylation. Acetylation, as the predominant form of histone acylation, is associated with the nuclear portion of several acetyl-CoA synthetases. Moreover, the interaction between nuclear acyl-CoA generating enzymes and acyltransferases enhances the efficiency of the acetylation process. While the regulatory mechanisms of metabolic enzyme localization in the nucleus are not yet fully understood, their roles in histone acetylation underscore the close connection between cellular metabolism and epigenetic regulation.
Untargeted Metabolomics and Proteomics Reveal Signatures of Post-Translational Modifications
Untargeted metabolomics and proteomics have played crucial roles in uncovering metabolic patterns under specific biological conditions, thereby aiding the formulation of preliminary hypotheses regarding related post-translational modifications (PTMs). For instance, untargeted metabolomics revealed the upregulation of undecanoic acid in human macrophages following lipopolysaccharide (LPS) treatment, as well as the metabolites from the polyamine biosynthesis pathway in mouse liver. These findings provide a foundation for exploring how the YAP/TAZ-polyamine-eIF5A oligomerization axis promotes the translation of histone demethylase LSD1. Additionally, untargeted metabolomics has provided insights into the role of STAT5 in acute myeloid leukemia (AML), revealing upregulation of glycolysis and lactate production in STAT5-activated cells, with lactate subsequently found to induce histone lactylation, favoring PD-1/PD-L1-based immunotherapy. Furthermore, untargeted metabolomics has uncovered changes in pyrimidine metabolism, offering insights into how O-GlcNAcylation regulates liver cancer growth.
In a typical untargeted metabolomics workflow, metabolite extracts are analyzed using high-performance liquid chromatography (HPLC), with most primary metabolites analyzed by hydrophilic interaction liquid chromatography (HILIC), and fatty acids or CoA derivatives analyzed by reverse-phase chromatography, complemented by mass spectrometry (MS). Untargeted analysis of the resulting large datasets aims to identify metabolites associated with specific biological conditions. While metabolite identification typically relies on compounds from standard databases or online spectral databases, more comprehensive analyses consider previously unidentified metabolites, utilizing tandem mass spectrometry (MS2) and other methods to identify all differential mass features. Recently, through untargeted metabolomic comparison of wild-type C. elegans and mitochondrial Sirtuin mutants, a series of previously unidentified metabolites, namely N-acyl spermidine, were discovered. Notably, the reduction in specific N-acyl spermidine abundance in mitochondrial Sirtuin mutants could indicate in vivo acyl substrates for Sirtuins, as demonstrated by studies in both C. elegans and mammalian cells.
Mass spectrometry-based proteomics is an indispensable tool for studying PTMs. However, due to the complexity of proteomic samples and current computational limitations, typical bottom-up proteomics workflows restrict the search for modified peptides to a short list of mass shifts. These limitations have driven the development of more advanced software tools, such as Byonic, which employs de novo sequencing strategies to allow the reporting of mass shifts derived from previously undefined PTMs. When analyzed in a comparative manner, Byonic reveals peptide clusters with common mass shifts that differ across biologically distinct groups, thus allowing for the further identification of the nature of these mass shifts. This approach has led to interesting biological discoveries.
In addition to improving the annotation of modified peptides, the quantification of modification stoichiometry is critical for prioritizing functional research sites. For example, chemical quantification analysis of liver proteomic samples from wild-type and Sirt5 knockout mice has identified SIRT5-regulated ammonia tolerance sites. However, it should not be assumed a priori that modifications with low stoichiometry are functionally irrelevant, as even low-stoichiometry phosphorylation can be sufficient to initiate signaling cascades. Establishing standards to prioritize modifications with low stoichiometry and understanding their physiological functions presents a major challenge, especially as innovations in mass spectrometry and bioinformatics may accelerate the detection of novel low-abundance PTMs.
In conclusion, advances in metabolomics and proteomics will continue to drive discoveries and further confirm the close relationship between metabolism, protein function, and PTM control, unveiling the complexity of cellular regulation.
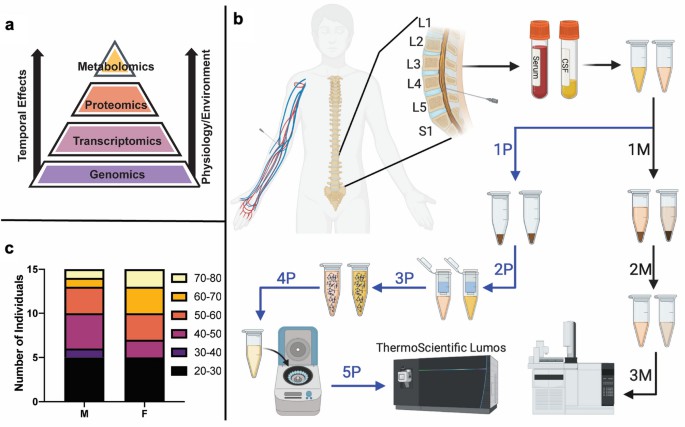 Figure 2 The metabolome and proteome are temporally more sensitive to environmental influence such as disease and injury.
Figure 2 The metabolome and proteome are temporally more sensitive to environmental influence such as disease and injury.
Conclusion
The diversity of protein modifications suggests the presence of complex regulatory mechanisms. Understanding these mechanisms, such as the contribution of metabolic enzymes to histone modifications, is crucial for understanding protein and site selectivity. The role of metabolite concentrations and localization within the cell, as well as the direct link between metabolism and protein modifications, is of significant importance for understanding metabolic regulation in processes such as immune responses and cancer progression.
Future research can consider exploring processes such as the transfer of mitochondrial metabolic enzymes to the nucleus, whether other nuclear-localized metabolic enzymes are involved in the modification of histones and other nuclear proteins, the effects of metabolic enzymes on modifications outside nuclear proteins, the selective factors of non-enzymatic metabolites in protein modifications, and the dynamic regulation of PTMs in the absence of enzyme clearance mechanisms.
References
- Zhang, B., Schroeder, F.C. Mechanisms of metabolism-coupled protein modifications. Nat Chem Biol (2025). https://doi.org/10.1038/s41589-024-01805-z
- Yawen Liu et al,. Metabolite-derived protein modifications modulating oncogenic signaling. 2022Sec. Molecular and Cellular Oncology. https://doi.org/10.3389/fonc.2022.988626
- Lilley, L.M., Sanche, S., Moore, S.C. et al. Methods to capture proteomic and metabolomic signatures from cerebrospinal fluid and serum of healthy individuals. Sci Rep 12, 13339 (2022). https://doi.org/10.1038/s41598-022-16598-1
Our products and services are for research use only.


.jpg)