Glycosylation, a formidable post-translational modification, meticulously modifies proteins, significantly influencing their morphology, functionality, and cellular positioning. This scholarly discourse takes an in-depth look into the sophisticated process of glycosylation, laying special emphasis on its two primary manifestations: the N-linked and O-linked glycosylation.
Glycosylation sites denote particular amino acid residues within a protein sequence where glycans are covalently affixed. Typically conforming to consensus sequences, they facilitate glycan attachment, predominantly occurring on amino acids such as asparagine (N), serine (S), and threonine (T).
What are N-Linked Glycosylation Sites
N-linked glycosylation sites are characterized by the attachment of glycans to asparagine residues within the Asn-X-Ser/Thr motif, where X represents any amino acid except proline. These sites are prevalent in proteins across all domains of life, contributing to structural stability and diverse functions.
Select Service
Mechanism of N-Linked Glycosylation
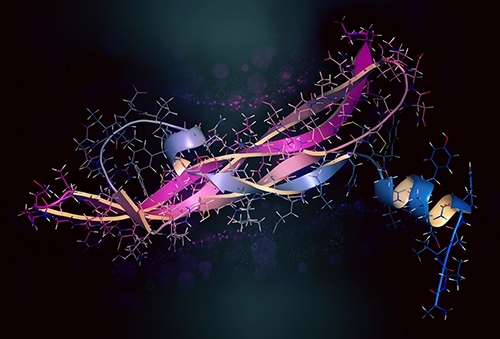
N-linked glycosylation, a co-translational process, intricately governs the attachment of glycans to asparagine residues within the Asn-X-Ser/Thr motif. This essential modification initiates during protein synthesis in the ER, where the nascent polypeptide chain is translocated into the ER lumen. Here, the oligosaccharyltransferase (OST) complex catalyzes the transfer of the preassembled oligosaccharide from a lipid carrier to the asparagine residue, forming a glycoprotein. This attachment occurs specifically when the consensus sequence Asn-X-Ser/Thr is located within the flexible loop regions of the protein, ensuring proper glycan accessibility and structural integrity (Kornfeld and Kornfeld, 1985). Following glycan transfer, the glycoprotein undergoes further processing in the Golgi apparatus, where additional sugar residues are added and trimmed by glycosidases and glycosyltransferases, yielding diverse glycan structures with unique functional properties (Helenius and Aebi, 2001). Notably, recent studies utilizing advanced imaging techniques, such as cryo-electron microscopy, have provided unprecedented insights into the structural dynamics of the OST complex during glycosylation, shedding light on the molecular mechanisms underlying this essential biological process (Lizak et al., 2011). These findings underscore the intricate interplay between glycosylation enzymes and protein substrates, driving the precise attachment of glycans and ensuring proper protein function and localization.
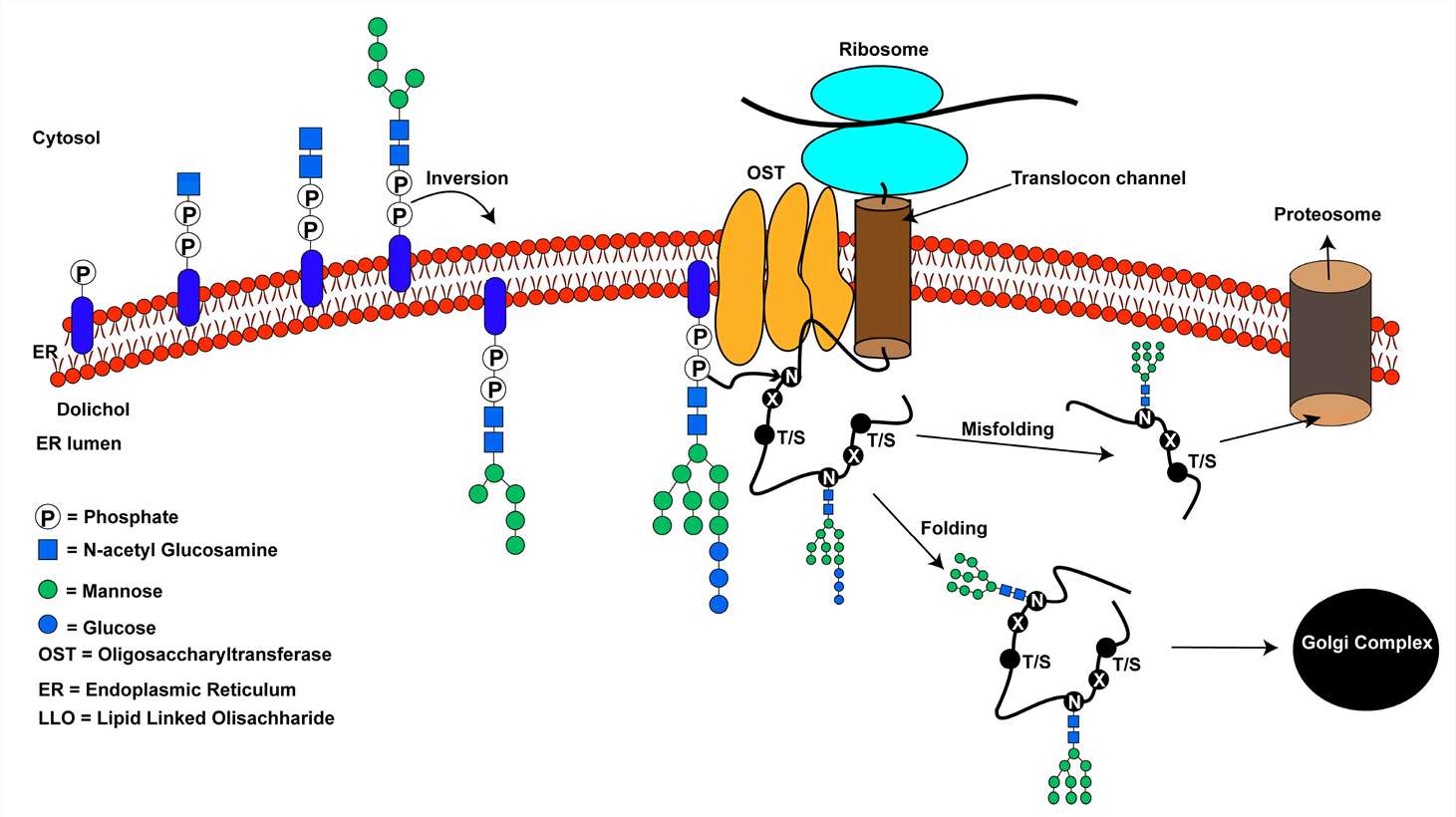 An overview of the N-linked glycosylation reaction of proteins in higher eukaryotes (Smita Mohanty et al,. Biomolecules 2020)
An overview of the N-linked glycosylation reaction of proteins in higher eukaryotes (Smita Mohanty et al,. Biomolecules 2020)
Structural Diversity of N-Linked Glycans
N-linked glycans showcase significant structural diversity, spanning from high-mannose to complex glycan forms. Central to N-linked glycosylation is the pentasaccharide Man3GlcNAc2, serving as a pivotal precursor for further glycan elaboration (Varki et al., 2017). High-mannose glycans, defined by terminal mannose residues lacking substitution, stand as primary structural motifs within N-linked glycans. These glycans typically comprise five to nine mannose units attached to the chitobiose core, with variations in mannose residue count contributing to their structural heterogeneity (Varki et al., 2015).
High-mannose glycans assume pivotal roles in protein folding and ER quality control processes, acting as recognition signals for ER-associated degradation (Aebi, 2013). Conversely, hybrid and complex glycans, elaborated in the Golgi apparatus, manifest heightened structural complexity and functional versatility. Hybrid glycans exhibit both unsubstituted and substituted mannose residues, forming a bridge between high-mannose and complex glycans (Moremen et al., 2012). Complex glycans, marked by GlcNAc additions at α-3 and α-6 mannose sites, epitomize N-linked glycan diversity, existing in bi-, tri-, and tetraantennary forms with varied branching patterns and terminal modifications (Varki et al., 2017). Structural analyses employing mass spectrometry and nuclear magnetic resonance spectroscopy unravel the heterogeneity and complexity of N-linked glycans, accentuating their significance across diverse biological processes (Moremen et al., 2012).
Biosynthesis and Degradation Pathways
The biosynthesis of N-linked glycans is intricately regulated, commencing at the cytoplasmic aspect of the ER and culminating within the Golgi apparatus. This orchestrated process relies upon a series of enzymatic reactions coordinated by glycosyltransferases, glycosidases, and other modifying enzymes (Moremen et al., 2012). Initially, glycan precursor assembly, termed dolichol pyrophosphate-linked oligosaccharide (LLO), takes place on a lipid carrier, dolichol phosphate, situated within the cytoplasmic leaflet of the ER membrane (Moremen et al., 2012; Aebi, 2013). This precursor, comprising Glc3Man9GlcNAc2 tethered to dolichol pyrophosphate, undergoes stepwise addition of monosaccharide units, typically initiated by N-acetylglucosamine (GlcNAc) and mannose residues transferred from nucleotide sugar donors such as UDP-GlcNAc and GDP-Man (Aebi, 2013). Upon completion, the LLO precursor is translocated across the ER membrane to its luminal side for further refinement and processing.
Within the ER lumen, the glycan precursor undergoes trimming and modification orchestrated by diverse glycosidases and glycosyltransferases. Sequential action of glucosidases and mannosidases progressively removes glucose and mannose residues, resulting in high-mannose-type N-linked glycans (Aebi, 2013). This trimming mechanism is pivotal for ER quality control, ensuring proper folding and maturation of glycoproteins. Subsequently, glycoproteins transit to the Golgi apparatus for further glycan remodeling. Golgi-resident glycosyltransferases sequentially add N-acetylglucosamine and other sugar residues, giving rise to hybrid and complex N-linked glycans (Moremen et al., 2012). This stepwise modification culminates in the generation of structurally diverse N-linked glycans, each endowed with unique functional properties.
Contrary to the construction of N-linked glycans, their degradation entails a collaborative effort of glycosidases that selectively target distinct glycosidic linkages within the glycan architecture. Notably, α-mannosidases and β-mannosidases act upon terminal mannose residues of high-mannose glycans, facilitating their exposure for subsequent processing or degradation (Varki et al., 2017). Additionally, sialidases and fucosidases play crucial roles in cleaving terminal sialic acid and fucose residues from complex N-linked glycans, respectively (Moremen et al., 2012). These exoglycosidases are pivotal constituents of glycan trimming and recycling pathways, thereby ensuring the turnover of glycoproteins and the preservation of cellular homeostasis.
Functions and Implications in Health and Disease
N-linked glycosylation is an instrumental factor in the realm of cellular physiology, exerting influence on diverse aspects such as protein folding, stability, and the mediation of critical signaling pathways which underpin organismal homeostasis. The integration of N-linked glycans to emerging polypeptide chains during protein biosynthesis is a supporting mechanism for appropriate folding, whilst simultaneously acting as a deterrent against misfolding or aggregation (Helenius and Aebi, 2001). This intricate process is enabled by the presence of chaperones residing within the ER, namely calnexin and calreticulin, which acknowledge glycan entities and facilitate protein maturation (Helenius and Aebi, 2001).
Furthermore, N-glycans localized on cellular surface receptors and adhesion molecules serve as regulators of immune responses, intercellular interactions, and tissue development (Varki et al., 2017). For instance, N-linked glycosylation modulates the activity of membrane receptors implicated in growth factor signaling and hormone responses, thereby dictating cellular behavior and function (Pinho and Reis, 2015).
Clinically, dysregulation in processes involving N-linked glycosylation contributes to pathology in various diseases. Genetic mutations affecting enzymes involved in glycosylation can lead to the development of congenital disorders of glycosylation, characterized by systematic dysfunction and accompanying developmental abnormalities. Additionally, tumor progression, metastasis, and immune system evasion can result from irregular glycosylation patterns seen in cancer cells. Besides, infectious diseases, certain pathogens demonstrate the ability to exploit host glycosylation machinery to enhance their virulence and inter-cellular interactions while evading immune surveillance. The need to understand the health and pathological roles of N-linked glycosylation remain pivotal in developing diagnostic strategies and targeted therapeutics for a host of pathological conditions.
What are O-Linked Glycosylation Sites
In the context of molecular biology, O-linked glycosylation sites denote the points of glycan linkage to serine or threonine residues inherent in a protein sequence. This is in contrast to the more prescriptive processes of N-linked glycosylation, which are governed by a consensus motif. The versatility characteristic of O-linked glycosylation originates from the absence of a stringent sequence prerequisite for glycan attachment. Such sites hold significant implications in an array of cellular processes that are fundamentally important, such as mediating interactions between cells, orchestrating cellular signaling pathways, and modulating the mechanisms of the immune system.
Select Service
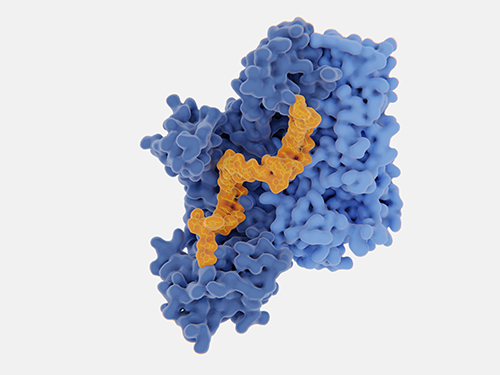
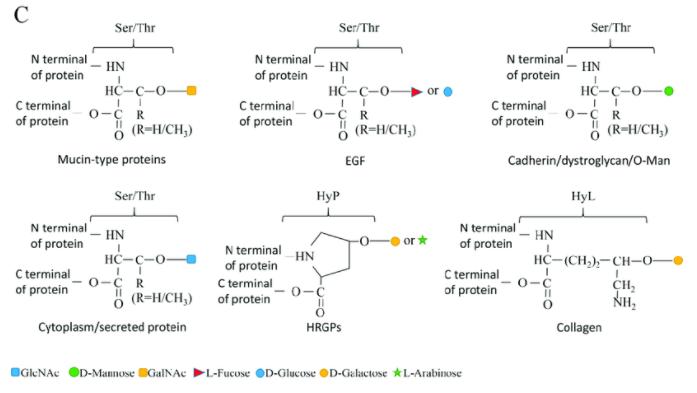 O-linked glycosylation types. (Borong Lin et al,. Cells 2020)
O-linked glycosylation types. (Borong Lin et al,. Cells 2020)
Mechanisms of O-Linked Glycosylation
Versatility of O-Linked Glycosylation Sites
Glycosylation sites exemplify extraordinary versatility, delineating a marked contrast to their N-Glycosylation counterparts by incorporating a wide array of potential attachment locations within protein sequences. In contrast to the defined consensus motif characteristic of N-linked glycosylation, O-linked glycosylation defies any specific sequence prerequisites, thus bestowing a heightened degree of flexibility with respect to glycan attachment (Varki et al., 2017). This adaptability originates from the attachment of glycans to the hydroxyl groups of serine or threonine amino acid residues within protein sequences, facilitating a broad spectrum of glycan structures and potential modifications. Hence, it can be concluded that O-linked glycosylation sites are not relegated to specific amino acid motif restrictions, providing a platform for glycan attachment at multifarious positions all along the protein sequence. This increased freedom in attachment locations amplifies the functional sophistication of O-linked glycans, granting them the capacity to modulate protein structure, stability, and functional responses amidst wide-ranging cellular environments.
Biosynthesis and Modification Processes
O-linked glycosylation demonstrates unrivaled versatility in contrast to its N-linked counterparts, thus enabling versatile locations for attachment within protein sequences. Deviating from the rigid consensus motif inherent to N-linked glycosylation, the O-linked glycosylation pathway does not mandate a prespecified sequence prerequisite, thereby increasing variability in glycan attachment points (Varki et al., 2017). This flexibility stems from attaching glycans to the hydroxyl groups present on serine or threonine residues within protein sequences. This mechanism facilitates the construction of a broad spectrum of glycan structures and modifications. As a result, O-linked glycosylation sites are not strictly tied to specific amino acid motifs, thereby fostering glycan attachment at manifold positions along the protein chain. This versatility bestowed upon attachment locations contributes to the functional intricacy of O-linked glycans, equipping them to manipulate protein configuration, stability, and utility across a diverse array of cellular environments.
O-Linked Glycosylation Implications in Disease Pathology
The pathological implications of aberrant O-linked glycosylation are profound and multidimensional. The misregulation of O-linked glycosylation is linked with an assortment of diseases, spanning cancer, inflammation, and neurodegenerative disorders. Within oncology, perturbed O-linked glycosylation profiles of cell surface receptors and adhesion molecules may augment tumor cellular growth, infiltration, and metastasis (Pinho and Reis, 2015). Moreover, improper glycosylation of proteins implicated in signaling pathways, such as growth factor receptors and cytokine receptors, can lead to an imbalance in cellular responses and spur tumor progression (Varki et al., 2017). Furthermore, the alteration of O-linked glycan architectures on the extracellular matrix components may perturb cell-matrix interactions and stimulate tumor angiogenesis and metastasis (Pinho and Reis, 2015).
Inflammation is another pathological state intertwined with misregulated O-linked glycosylation. The altered glycosylation of surface receptors and adhesion molecules of immune cells may modulate the activation, migration, and cytokine production of these cells, ensuing chronic inflammation and tissue degradation (Rabinovich et al., 2012). Additionally, the aberrant O-linked glycosylation of inflammatory mediators, namely cytokines and chemokines, may influence their bioactivity, thereby contributing to the pathogenesis of inflammatory ailments (Varki et al., 2017).
Evocatively, accumulated evidence postulates a link between abnormal O-linked glycosylation and neurodegenerative disorders, such as Alzheimer's (AD) and Parkinson's disease (PD). Detectable glycosylation abnormalities in neuronal proteins, inclusive of tau and α-synuclein, have been identified in AD and PD brain tissues, respectively (Arnold et al., 2017; Hasegawa et al., 2002). These glycosylation changes may influence protein aggregation, neuronal functionality, and synaptic signaling, cumulatively contributing to neurodegeneration and cognitive deterioration (Arnold et al., 2017; Hasegawa et al., 2002).
In summation, dysregulated O-linked glycosylation can markedly impact cellular processes integral to disease pathology, such as cell proliferation, inflammation, and neuronal function. The comprehension of the molecular mechanics underpinning aberrant glycosylation in disease conditions remains crucial to the forging of novel therapeutic strategies aimed at targeting glycan-centric pathways.
N-linked Glycosylation vs. O-linked Glycosylation: Contrasts and Comparisons
| Aspect | N-linked Glycosylation | O-linked Glycosylation |
|---|---|---|
| Mechanism and Attachment Sites | Involves the attachment of glycans to asparagine residues within the Asn-X-Ser/Thr motif. Initiates during protein synthesis in the endoplasmic reticulum, with further modification in the Golgi apparatus. Adheres to a strict consensus sequence. | Involves the attachment of glycans to serine or threonine residues within protein sequences. Lacks a strict consensus motif, offering versatility in glycan attachment. Predominantly occurs in the Golgi apparatus. |
| Structural Diversity | Exhibits structural diversity, ranging from high-mannose to complex forms. Common core pentasaccharide, Man3GlcNAc2, serves as the foundation for further glycan elaboration. Additional sugars sequentially added and trimmed, resulting in diverse glycan structures. | Exhibits structural diversity, with varying degrees of glycan branching and modification. Glycans play essential roles in modulating protein structure, stability, and function. Absence of a consensus sequence allows for diverse glycan structures. |
| Functional Implications | Plays crucial roles in protein folding, stability, and intracellular trafficking. Modulates protein-protein interactions, cell adhesion, and signaling pathways. Influences diverse cellular processes, including immunity, development, and disease progression. Dysregulation associated with congenital disorders of glycosylation (CDGs) and various diseases. | Involved in a wide range of cellular processes, including cell-cell interactions, extracellular matrix organization, and signaling cascades. Regulates cell adhesion, migration, and tissue development. Dysregulation implicated in cancer, inflammation, and neurodegenerative disorders. |
| Consensus Sequence | Follows a strict consensus motif (Asn-X-Ser/Thr), ensuring precise glycan attachment and structural integrity. | Lacks a defined sequence requirement, offering versatility in glycan attachment. |
| Glycan Complexity | Larger and more complex, often exhibiting greater structural heterogeneity. | Generally smaller and less complex, with fewer branching patterns and structural variations. |
| Enzymatic Machinery | Involves a series of glycosyltransferases and glycosidases in the endoplasmic reticulum and Golgi apparatus. | Requires a distinct set of glycosyltransferases and glycosidases localized predominantly in the Golgi apparatus. |
| Recognition Motifs | Recognizes the Asn-X-Ser/Thr motif, where X can be any amino acid except proline. | Lacks a specific consensus motif but may occur in regions rich in serine and threonine residues. |
| Spatial Localization | Prevalent in secretory and membrane-bound proteins, as well as extracellular proteins. | More commonly found in extracellular proteins, mucins, and proteins localized on cell surfaces. |
| Role in Disease | Mutations or dysregulation associated with congenital disorders of glycosylation (CDGs) and various diseases, including cancer and neurodegenerative disorders. | Aberrant O-linked glycosylation implicated in cancer progression, inflammatory diseases, and certain neurodegenerative disorders. |
| Regulation | Tightly regulated by the availability of glycosylation substrates, enzymatic activity, and cellular signaling pathways. | Modulated by cellular signaling events, extracellular stimuli, and changes in protein conformation. |
Conclusion
In summary, Protein glycosylation, encompassing both N-linked and O-linked processes, significantly diversifies the structure and functionality of glycoproteins. N-linked glycosylation, occurring in a sequenced manner and undergoing intricate processing in the endoplasmic reticulum and Golgi apparatus, contributes to protein folding, stability, and cellular trafficking. Conversely, O-linked glycosylation offers flexibility in attachment sites, influencing various cellular processes such as intercellular interactions and signaling. Both types of glycosylation are integral to immunity, development, and disease progression, with dysregulation associated with numerous pathologies. Understanding glycan biosynthesis and modifications is essential for developing therapeutic strategies targeting glycan-related pathways and advancing biomedical research.
References
- Kornfeld, R., & Kornfeld, S. (1985). Assembly of asparagine-linked oligosaccharides. Annual Review of Biochemistry, 54(1), 631-664.
- Helenius, A., & Aebi, M. (2001). Intracellular functions of N-linked glycans. Science, 291(5512), 2364-2369.
- Lizak, C., Gerber, S., Numao, S., Aebi, M., & Locher, K. P. (2011). X-ray structure of a bacterial oligosaccharyltransferase. Nature, 474(7351), 350-355.
- Aebi, M. (2013). N-linked protein glycosylation in the ER. Biochimica et Biophysica Acta (BBA)-General Subjects, 1833(11), 2430-2437.
- Moremen, K. W., Tiemeyer, M., & Nairn, A. V. (2012). Vertebrate protein glycosylation: diversity, synthesis and function. Nature Reviews Molecular Cell Biology, 13(7), 448-462.
- Varki, A., Cummings, R. D., Esko, J. D., Stanley, P., Hart, G. W., Aebi, M., ... & Packer, N. H. (2017). Essentials of Glycobiology. Cold Spring Harbor Laboratory Press.
- Varki, A., Cummings, R. D., Esko, J. D., Stanley, P., Hart, G. W., Aebi, M., Darvill, A. G., Kinoshita, T., Packer, N. H., Prestegard, J. H., Schnaar, R. L., and Seeberger, P. H. (2015). Symbol nomenclature for graphical representations of glycans. Glycobiology, 25(12), 1323-1324.
- Pinho, S. S., and Reis, C. A. (2015). Glycosylation in cancer: mechanisms and clinical implications. Nature Reviews Cancer, 15(9), 540-555.
- Rabinovich, G. A., van Kooyk, Y., and Cobb, B. A. (2012). Glycobiology of immune responses. Annals of the New York Academy of Sciences, 1253(1), 1-15.
- Arnold, J. N., Wormald, M. R., Sim, R. B., Rudd, P. M., and Dwek, R. A. (2007). The impact of glycosylation on the biological function and structure of human immunoglobulins. Annual Review of Immunology, 25, 21-50.
- Hasegawa, M., Tsutsumi, K., Nakagawa, T., Iwatsubo, T., and Yamaguchi, M. (2002). [Structural role of N-glycans in intracellular retention of Alzheimer's amyloid precursor protein expressed in non-neuronal cells]. Seikagaku. The Journal of Japanese Biochemical Society, 74(4), 411-417.
Our products and services are for research use only.