Protein phosphorylation, a pivotal mechanism of cellular signal transduction, involves the transfer of phosphate groups by kinases to amino acid residues of proteins, thereby modulating their function. This post-translational modification can alter protein structure and activity, consequently influencing various physiological processes such as cellular metabolism, proliferation, and differentiation. Protein phosphorylation plays an indispensable role in numerous biological processes and is crucial for maintaining normal cellular functions. In biomedical research, Western Blotting is a fundamental technique that researchers must master. Although many researchers are quite familiar with Western Blotting, they often encounter challenges and confusion when dealing with phosphorylated proteins. This is due to the rapid responsiveness, susceptibility to degradation, and low expression levels characteristic of phosphorylated proteins. When performing Western Blot analysis of phosphorylated proteins, special attention must be paid to several technical details, distinguishing it from conventional Western Blotting. This paper provides an in-depth exploration of considerations essential for Western Blot analysis of phosphorylated proteins.
Protein phosphorylation
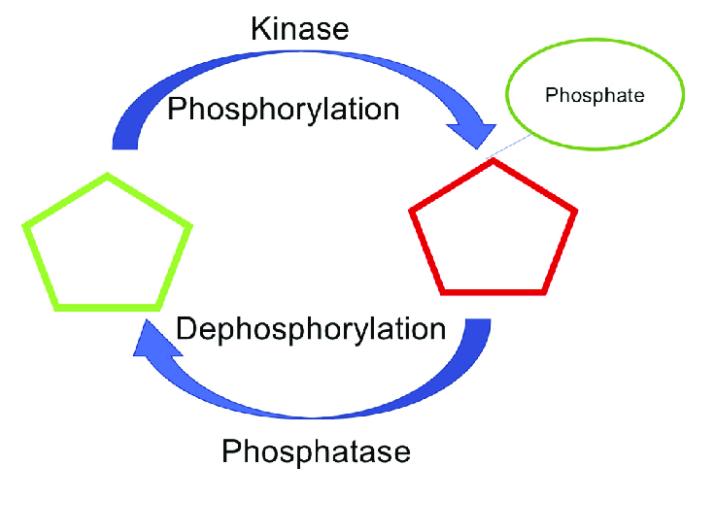
Protein phosphorylation modification is a ubiquitous and reversible biochemical reaction, involving the transfer of a phosphate group from adenosine triphosphate (ATP) to the side chains of amino acids within proteins. This modification is predominantly catalyzed on serine, threonine, and tyrosine residues and can be reversed by the action of phosphatases. The phosphorylation of proteins markedly influences their three-dimensional conformation and biological activity, thereby regulating key physiological processes such as cellular metabolism, proliferation, and differentiation. As a fundamental mechanism for modulating protein activity and function, the significance of phosphorylation modification cannot be overstated. Current research on phosphorylation modifications is primarily focused on understanding disease mechanisms, cell signal transduction, drug development, developmental biology, and stress responses, among other areas.
Challenges in Western Blotting of Phosphorylated Proteins
Firstly, the abundance of phosphorylated proteins is generally low, accounting for approximately 10% or even less of the total protein content. This low proportion makes the detection and observation of phosphorylated proteins relatively challenging during experimental procedures. Secondly, the phosphorylation modification of proteins is inherently reversible. Upon cell lysis, the release of proteases and phosphatases can result in the rapid dephosphorylation of phosphorylated proteins, often occurring within milliseconds. Consequently, it becomes a significant challenge to capture or maintain the stability of phosphorylated proteins throughout the experimental process.
Preparation Before the Experiment: Comprehensive Understanding of the Research Subject
Prior to commencing experiments, it is critical to gain an in-depth understanding of the intrinsic properties and context of the protein of interest. Researchers typically achieve this through a review of existing literature or preliminary experiments to verify the expression of phosphorylated proteins at the target sites within their samples. In this process, the use of the PhosphoSitePlus database is highly recommended. This comprehensive resource is specifically designed for the prediction of protein post-translational modifications. By inputting the name of a particular protein, researchers can access information regarding various modification types, modification site details, and other relevant data. If the phosphorylation levels in the samples are inherently low or absent under normal conditions, physical or chemical stimulation may be required. Appropriate stimulation is crucial for obtaining optimal experimental outcomes, and the duration and intensity of such stimulation must be precisely determined through literature review and preliminary experimentation.
 The phosphorylation status of specific protein sites can be investigated by querying the PhosphoSitePlus database (https://www.phosphosite.org).
The phosphorylation status of specific protein sites can be investigated by querying the PhosphoSitePlus database (https://www.phosphosite.org).
Sample Processing
- The freshness of the samples is critical for the accuracy of experimental outcomes; therefore, it is recommended that samples be processed immediately after collection.
- During handling, procedures should be carried out swiftly and efficiently to minimize external interference with the samples.
- Reagents and equipment must be pre-cooled, preferably using an ice bath, to ensure the stability of the reagents.
- During cell or tissue disruption, the use of ultrasonic disintegrators should be avoided. For tissue grinding, it is advisable to pre-cool the mortar with liquid nitrogen.
- Protease and phosphatase inhibitors should be added to the lysis buffer to preserve the integrity of the target molecules.
- It is crucial to note that boiling should be avoided for certain phosphorylated proteins, as this can lead to the degradation of phosphorylation sites.
- After determining sample concentration, loading buffer should be added, and aliquoted samples should be immediately frozen at -80°C to prevent the adverse effects of repeated freeze-thaw cycles on sample quality during long-term storage.
Internal Controls
In experimental research, it is customary to establish two internal controls. Firstly, the ratio of phosphorylated protein to total protein serves as a crucial metric. This ratio is essential because the measurement of changes in phosphorylated protein expression alone may not comprehensively address the research question. Only when there is a variation in this ratio can changes in phosphorylation levels be accurately reflected. Secondly, an additional internal control is utilized to validate the reliability of the experimental results within the system.
Significant errors can occur during well-to-well comparisons. To minimize such errors, samples are often treated with stripping solutions to remove primary and secondary antibodies initially bound to phosphorylated proteins. Subsequently, the same membrane is used to probe for total protein. Following this, thorough washing and internal standard detection are performed to ensure the precision of the experimental results.
Loading and Blocking
In the context of Western Blot (WB) analysis for phosphorylated proteins, it is imperative to ensure an adequate sample loading, given the generally low expression levels of these proteins. Concurrently, high background signals represent a noteworthy concern in the detection of phosphorylated proteins. For instance, casein present in milk can contribute to background signal interference. Therefore, it is advisable to employ bovine serum albumin (BSA) or protein-free blocking agents to mitigate background noise. Additionally, if the antibody manufacturer's protocol provides specific experimental recommendations, these should be prioritized to ensure the accuracy and reliability of the experimental outcomes.
Antibody Incubation and Washing
1. Antibody Incubation Process
During experimental procedures, the steps of antibody incubation and washing play critical roles. To ensure optimal binding of the primary antibody specific for phosphoproteins, it is recommended to perform an overnight incubation at 4°C. This method provides sufficient time for antigen-antibody interactions and effectively prevents the dissociation of the protein antigens from the membrane. For secondary antibody incubation, a one-hour incubation at room temperature is advisable to facilitate complete reaction.
2. Washing Procedure Optimization
It is suggested to moderately reduce the frequency of wash cycles and decrease the shaking speed of the platform. Although background signals may compromise the clarity of experimental results, their presence at least confirms the presence of reactive substances within the samples. Subsequent experiments can be appropriately adjusted and optimized from this foundation to achieve enhanced experimental outcomes.
Experimental Protocol Considerations
Principles of "Rapid, Accurate, and Low-Temperature" in Phosphoprotein Extraction
The principle of "rapid, accurate, and low-temperature" is paramount in the extraction of phosphoproteins. "Rapid" refers to the efficiency in sample preparation and operational procedures. "Accurate" pertains to the precise execution of experimental steps and the exact addition of reagents. "Low-temperature" emphasizes conducting the entire experiment on ice, with all reagents and equipment pre-cooled to minimize the risk of phosphoprotein degradation. It is imperative to incorporate freshly prepared protease and phosphatase inhibitors to comprehensively inhibit protease and phosphatase activities. The extracted phosphoproteins should be promptly denatured and aliquoted for storage at -80°C to prevent degradation of phosphate groups due to repeated freeze-thaw cycles. Additionally, to discern whether observed changes in phosphorylation levels are due to alterations in phosphorylation status or protein expression levels, it is essential to include total protein references, alongside commonly used internal controls such as Actin and GAPDH, to enable accurate analysis of results.
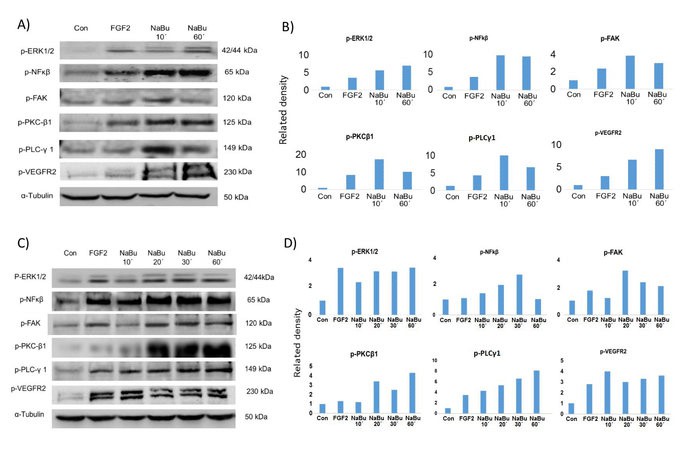 Western blotting shows phosphorylated proteins (Mark Slevin et al,.2016)
Western blotting shows phosphorylated proteins (Mark Slevin et al,.2016)
Protein phosphorylation
Considerations in Western Blot Experimental Procedures
Following protein quantification, samples should be promptly mixed with loading buffer to inhibit phosphatase activity. Subsequently, these samples can be aliquoted and stored in a low-temperature freezer or directly utilized for Western Blot analysis. To ensure the accuracy of experimental results, prolonged storage of samples should be avoided. It is recommended that samples be prepared freshly for immediate use.
Given that phosphorylated proteins often constitute a relatively low proportion of the total protein pool, accounting for approximately 10%, it is advisable to maximize the loading volume during experiments to mitigate potential adverse effects on result interpretation and analysis. Particularly, it is crucial to avoid boiling certain phosphoprotein samples prior to loading to prevent disruption of phosphorylation sites.
During the blocking step, casein, a major component of milk, can contribute to elevated background signals. Therefore, when detecting phosphorylated proteins, it is recommended to use bovine serum albumin (BSA) or a protein-free blocking agent, and to limit the blocking time to 1-1.5 hours.
To minimize nonspecific signals, it is advisable to employ Tris-Buffered Saline with Tween 20 (TBST) instead of Phosphate-Buffered Saline (PBS). Should PBS be necessary during the experiment, a thorough washing of the membrane with copious amounts of TBST should be conducted prior to the addition of the protein detection substrate, ensuring the removal of excess sodium phosphate.
Common Issues in Phosphoprotein Western Blot Detection
Issue 1: Loading Volume of 25 μL and Blocking with 5% Milk for 1 Hour, Resulting in Blurred Bands and High Background Staining
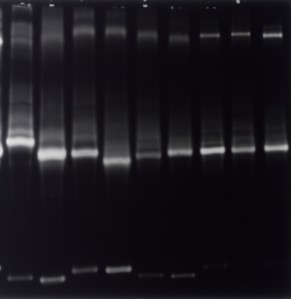
Recommendation: The small loading volume necessitates extended exposure times, thereby intensifying background staining. Furthermore, the phosphoproteins present in milk may exacerbate background interference. It is advised to increase the loading volume to 40 μL and utilize 5% bovine serum albumin (BSA) or serum for blocking purposes.
Issue 2: Prolonged Storage of Samples at -20°C with a Loading Volume of 20 μL, Resulting in Severe Non-specific Bands and Indistinct Target Bands with High Background Staining
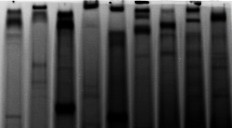
Recommendation: The small loading volume requires prolonged exposure time, thus increasing background intensity. Long-term storage may have led to the degradation of phosphoproteins, making target bands undetectable. It is recommended to re-extract the proteins, increase the loading volume, and repeat the Western Blot analysis.
Issue 3: Detection Using Unstimulated Hela Cells Stored at -20°C for One Week with a Loading Volume of 20 μL, Resulting in Absence of Bands
Analysis and Improvement Suggestions: The phosphorylation level of the protein site in unstimulated Hela cells is inherently low. This, combined with insufficient loading volume and extended storage times, affects experimental outcomes. Treating samples with PMA, immediately extracting proteins, and conducting Western Blot analysis is recommended for obtaining clear and optimal results.
Conclusion
Successful detection of phosphorylated proteins via Western Blot is contingent upon comprehensive preliminary research and meticulous experimental execution. The value of pilot experiments lies in allowing researchers to fine-tune critical steps such as protein extraction, electrophoresis, membrane transfer, and immunoblotting. This process aids in optimizing experimental conditions and establishing the optimal antibody concentration and working parameters, thereby ensuring precise detection of target proteins. Such attention to detail is pivotal for accurate interpretation of experimental data and significantly influences the precision and reliability of research findings.
Reference
- Pan B, Zhu X, Han B, Weng J, Wang Y, Liu Y. The SIK1/CRTC2/CREB1 and TWIST1/PI3K/Akt/GSK3β signaling pathways mediated by microRNA-25-3p are altered in the schizophrenic rat brain. Front Cell Neurosci. 2023 Jan 20;17:1087335. DOI: 10.3389/fncel.2023.1087335. PMID: 36744005; PMCID: PMC9896578.
Our products and services are for research use only.