Lactylation is an important post-translational modification process. This article discusses the occurrence of lactylation, the commonly used research methods, and its significant roles in driving gene expression, epigenetic regulation, metabolic regulation, signal transduction, immune modulation, and the progression of various diseases, including cancer, neurodegenerative diseases, and cardiovascular diseases.
Driving Gene Expression
Lactylation is becoming recognized as a key regulator in the transcription of crucial genes, particularly through its impact on histone post-translational modifications. One of the most prominent sites for lactylation is H3K18, a modification that plays a vital role in transcription elongation, influencing the expression of multiple housekeeping genes. This specific lactylation event is not just a fleeting modification—it serves as a hallmark of active promoters and is notably found in tissue-specific enhancers. Beyond H3K18, lactylation can also target other histone sites like H2AK11, H2BK16, H2BK120, H3K9, H3K14, H3K23, H3K56, H4K5, H4K12, and H4K16, which collectively regulate the expression of downstream genes, further highlighting the diverse role of lactylation in gene expression.
Interestingly, the availability of lactylation antibodies for many of these histone sites has opened doors for researchers to probe deeper into the mechanisms governing gene expression and cell function. Lactylation isn't confined to histones alone—its influence stretches to transcription factors and non-coding RNAs (ncRNAs). A notable example is lactylation at Lys183 on the transcription factor YY1, which boosts the transcription of FGF2, thereby enhancing angiogenesis. Additionally, the circular non-coding RNA CircXRN2 can regulate H3K18 lactylation levels, thereby modulating gene expression indirectly. These findings emphasize the broad and intricate impact lactylation has on cellular regulation, extending its reach from histones to transcription factors and ncRNAs.
Such discoveries open up exciting avenues for exploring lactylation's role in vital physiological processes, from neuroendocrine functions to fibrosis, and even macrophage reparative activity. It's clear that lactylation isn't just a minor player—it's a major force driving gene expression and influencing various cellular responses.
Influencing Protein Function
Lactylation, as a post-translational modification, can directly impact the conformation and function of proteins. Studies have shown that lactylation stabilizes proteins, such as lymphocyte cytosolic protein 1 (LCP1), β-catenin, and hypoxia-inducible factor-1α (HIF-1α), protecting them from degradation and allowing them to maintain their function.
Lactylation can also affect protein-protein interactions. For example, lactylation at Lys1897 of α-MHC reduces its interaction with Titin. Additionally, lactylation of the phosphoinositide 3-kinase catalytic subunit p110δ enhances its interaction with Beclin1, thereby increasing the activity of this lipid kinase.
In terms of the activity of metabolism-related enzymes, lactylation may act as a feedback regulator of glycolysis by affecting enzyme structure. For instance, lactate produced during glycolysis increases lactylation at Lys62 of pyruvate kinase M2 (PKM2), inhibiting the dimer-to-tetramer transition of PKM2, reducing its nuclear distribution, and enhancing its activity. Lactylation also regulates the distribution of metabolic enzymes, contributing to the overall regulation of metabolism in organisms.
Interactions Between Lactylation and Other Modifications
The interaction between protein lactylation and other PTMs, such as phosphorylation and acetylation, is a complex and critical area of research. These modifications can influence each other and cooperate to regulate the structure, function, and activity of proteins. Recent studies have shown that elevated acetylation levels and the inactivation of PDHA1 promote lactate accumulation, which in turn facilitates the lactylation of mitochondrial fission protein 1. This process may exacerbate damage to renal tubular epithelial cells and worsen acute kidney injury.
Lactylation and acetylation, two PTMs, may not only interact at the molecular level to jointly participate in the complex regulatory networks within cells, but they can also play synergistic or antagonistic roles in various biological processes. Acetylation involves the transfer of an acetyl group, while lactylation adds a lactyl group to specific amino acid residues of proteins, usually lysine. Therefore, these modifications may compete at the same modification sites, affecting the functional state of proteins. Given that the modification status of proteins can serve as a "signal switch" for multiple biological processes within cells, this competitive relationship may also involve broader mechanisms of cellular function regulation and environmental adaptation.
Furthermore, the substrates for lactylation are primarily lactate, while the substrates for acetylation are mainly acetyl-CoA. Both substrates can be generated through different metabolic reactions starting from pyruvate. Pyruvate produces lactate through glycolysis in the cytoplasm, while it generates acetyl-CoA when entering the mitochondria. Any changes in these metabolic activities could disrupt the original balance between lactylation and acetylation. Such an imbalance could affect signal transduction, functional regulation, and feedback regulation of metabolic activities, ultimately having a significant impact on cellular fate.
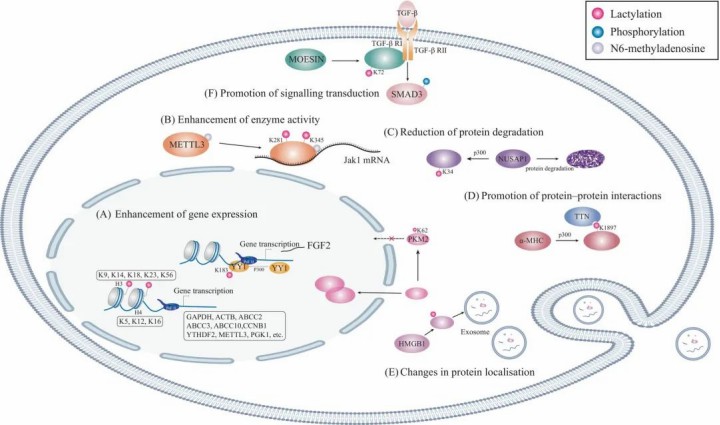 Figure 1: Lactylation Modifications in Biological Functions
Figure 1: Lactylation Modifications in Biological Functions
Select Service
More Resources
Lactylation Affects the Progression of Various Diseases
Current research indicates that lactylation is associated with the onset and progression of several diseases, including cancer, neurodegenerative diseases, cardiovascular diseases, and other conditions.
Cancer
Lactylation is closely linked to the occurrence and progression of tumors. It was first identified in liver cancer cells, and subsequent studies have revealed its role in metabolic pathways related to tumors, such as the TCA cycle, carbohydrate, amino acid, fatty acid, and nucleotide metabolism. The role of lactylation in various cancers, including gastric cancer, prostate cancer, and ocular melanoma, has been extensively studied and is associated with poor prognosis.
Lactylation affects cancer progression through multiple mechanisms:
Promoting oncogene expression and affecting immune cell function in the tumor microenvironment (TME), leading to immunosuppression and immune evasion.
Enhancing histone lactylation in tumor-associated macrophages, correlating with the M2 phenotype and potentially contributing to tumor formation and malignant progression.
Modulating cellular plasticity and neuroendocrine differentiation in prostate and lung adenocarcinomas.
Neurodegenerative Diseases
Lactylation is widely present in the brain and plays a crucial role in regulating chromatin states and gene expression. It may be influenced by neuronal excitation and social stress, and is linked to reduced social behavior and increased anxiety-like behaviors.
Recent findings include:
Lactate modifies specific lysines on amyloid precursor protein (APP), altering its intracellular transport and degradation.
This modification limits production and aggregation of Aβ in mice.
L-lactate injections slow amyloid accumulation and preserve memory in AD mice.
Lactylation also plays a role in other neurodegenerative diseases, such as Parkinson's disease, multiple sclerosis, amyotrophic lateral sclerosis, and Huntington's disease
Cardiovascular Diseases
Lactylation plays multiple roles in the recovery process following myocardial infarction. On one hand, it may exacerbate cardiac dysfunction by activating the TGF-β signaling pathway, promoting cardiac fibrosis; on the other hand, it may aid in cardiac repair by promoting the early activation of monocytes. In cerebral infarction, lactylation mediates neuronal death through its effect on mitochondrial apoptosis pathways, hindering recovery after ischemia-reperfusion injury.
Other Diseases
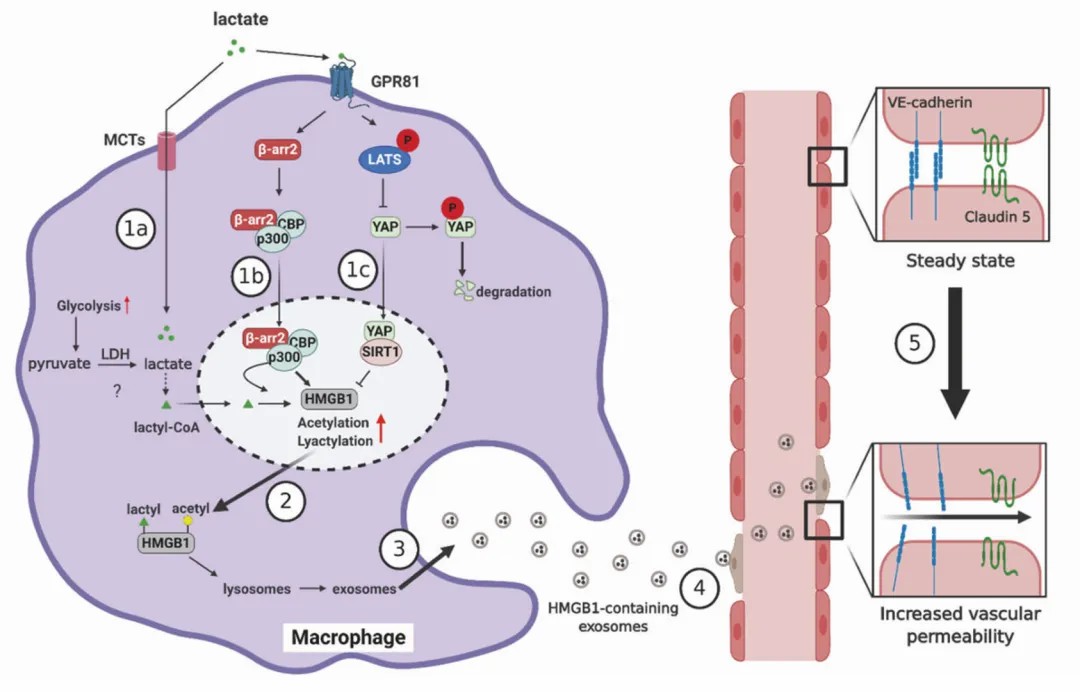
Lactylation has also garnered widespread attention in developmental abnormalities, metabolic disorders, and inflammatory diseases. In developmental abnormalities, lactylation may affect the meiotic process in mouse oocytes and the implantation of the endometrium. In metabolic diseases, lactylation is associated with obesity and insulin resistance, potentially influencing metabolic pathways in skeletal muscle and white adipose tissue. In inflammatory and infectious diseases, lactylation is closely related to macrophage differentiation and may affect the expression of tissue repair genes.
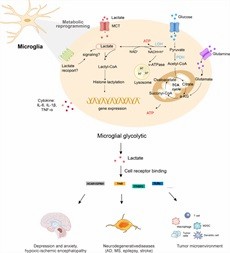 Figure 2: Mechanisms of microglia lactylation in relation to disease regulation.
Figure 2: Mechanisms of microglia lactylation in relation to disease regulation.
Prospects of Lactylation in Clinical Applications
Changes in lactylation levels can serve as biomarkers for early disease diagnosis and disease progression monitoring. Especially in cancers, metabolic disorders, and inflammatory diseases, bioinformatics tools can leverage big data analysis and lactylation status information to build more effective diagnostic and detection models, helping with disease prevention and treatment.
Targeted therapy is gaining increasing attention in clinical practice, and identifying specific and effective drug targets is crucial. Drug development targeting lactylation mainly focuses on small molecule inhibitors of MCT1 (which affect lactate transport) and LDH inhibitors (which block lactate production). Future research should explore the molecular mechanisms of lactylation and identify molecules that regulate lactylation.
Analyzing individual differences in lactylation and exploring its application in personalized medicine can provide patients with customized treatment plans, serving as an important approach for achieving precision treatment. However, lactylation still presents challenges in functional studies, as it may be influenced by individual variability. The application of lactylation in personalized medicine still requires extensive clinical trials and validation to prove its effectiveness and feasibility.
References
- Jing F, Zhang J, Zhang H, Li T. Unlocking the multifaceted molecular functions and diverse disease implications of lactylation. Biol Rev Camb Philos Soc. 2024 Sep 16. doi: 10.1111/brv.13135.
- Tian Q, Li J, Wu B, Pang Y, He W, Xiao Q, Wang J, Yi L, Tian N, Shi X, Xia L, Tian X, Chen M, Fan Y, Xu B, Tao Y, Song W, Du Y, Dong Z. APP lysine 612 lactylation ameliorates amyloid pathology and memory decline in Alzheimer's disease. J Clin Invest. 2025 Jan 2;135(1) PubMed.
- Hu Y, He Z, Li Z, Wang Y, Wu N, Sun H, Zhou Z, Hu Q, Cong X. Lactylation: the novel histone modification influence on gene expression, protein function, and disease. Clin Epigenetics. 2024 May 29;16(1):72. doi: 10.1186/s13148-024-01682-2. PMID: 38812044; PMCID: PMC11138093.
- Yang, Hui1; Mo, Nan2; Tong, Le3; Dong, Jianhong1; Fan, Ziwei4; Jia, Mengxian4; Yue, Juanqing5,*; Wang, Ying6,*. Microglia lactylation in relation to central nervous system diseases. Neural Regeneration Research 20(1):p 29-40, January 2025. DOI: 10.4103/NRR.NRR-D-23-00805
Our products and services are for research use only.