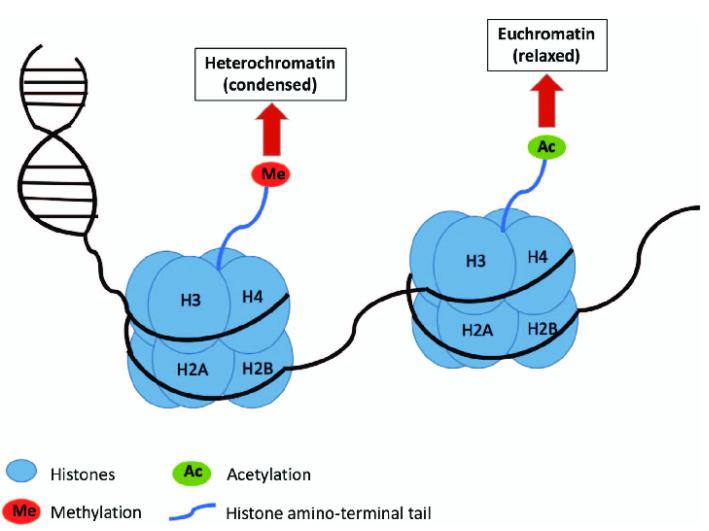
Interplay between Lactylation and Other Lysine Acylation Modifications
Cellular metabolism produces a plethora of intermediate metabolites that can serve as feedback and feedforward regulators of post-translational modifications (PTMs). Among these metabolites, lactate, a byproduct of glycolysis, mediates the interplay between glycolytic processes, lactate accumulation, and the lactylation of proteins. Numerous studies have established the critical role of protein lactylation in various human diseases related to cellular fate determination, embryonic development, inflammation, tumors, and neuropsychiatric disorders. Further investigations have confirmed that protein lactylation is essential for glucose metabolism and the regulation of glycolytic pathways. Additionally, during pathological processes such as inflammation, fibrosis, and cancer, protein lactylation exhibits diverse functional roles.
With advancements in liquid chromatography-tandem mass spectrometry (LC-MS/MS) and the expanding variety of lysine acylation types, an increasing number of studies have elucidated the similarities in lysine acylation regarding substrate proteins, catalytic enzymes, physiological functions, and associated diseases. Lysine acylation is dynamically regulated by a balance between "writer" and "eraser" enzymes. Within the histone acetyltransferase (HAT) family, p300 is recognized as one of the most versatile acetyltransferases identified to date, possessing the capacity to catalyze various acyl modifications, including butyryl, propionyl, isobutyryl, β-hydroxybutyryl, 2-hydroxyisobutyryl, lactyl, crotonyl, and isonicotinyl modifications. In terms of "eraser" mechanisms, several lysine deacetylases (KDACs), such as HDAC1-3, have been found to play extensive roles across different acyl modifications.
Consequently, a coordinated or competitive interplay may exist among acetylation, lactylation, and crotonylation, thereby shaping the overall modification landscape of specific proteins. Moreover, lysine acylation not only occurs through the action of acyltransferase "writer" enzymes but may also be mediated by non-enzymatic mechanisms. For instance, a pH-dependent process favors increased acetylation at elevated pH levels. The high proton production associated with glycolytic lactate metabolism contributes to intracellular acidification, which may act as a regulator of non-enzymatic protein acetylation.
Furthermore, lactate has been shown to inhibit HDAC activity, thus promoting alterations in gene expression. This inhibitory effect of lactate on HDACs constitutes an additional mechanism underlying the PTM interplay between lactate-dependent modifications and other lysine acylation events.
In summary, the multifaceted roles of lactylation in cellular processes and its interaction with other lysine acylation types underscore the complexity of metabolic regulation in health and disease. Future research should focus on elucidating the specific molecular mechanisms governing these interactions to better understand their implications for cellular function and therapeutic strategies.
Case Study
Case Study 1: Lactate Promotes Macrophage HMGB1 Lactylation, Acetylation, and Exosomal Release in Polymicrobial Sepsis
Journal: Cell Death & Differentiation
Publication Date: August 2021
Background
Sepsis is a syndrome characterized by organ dysfunction resulting from a dysregulated response to infection. Prior studies have established a positive correlation between elevated serum lactate levels and mortality in sepsis. High mobility group box-1 (HMGB1) is a ubiquitous nuclear protein released by activated macrophages to coordinate inflammatory responses. Clinical evidence indicates that circulating HMGB1 levels are significantly elevated and positively correlated with the severity and mortality of sepsis. This study investigates whether lactate can promote the lactylation and acetylation of HMGB1 in macrophages during polymicrobial sepsis.
Main Findings
During polymicrobial septic infection, macrophages uptake extracellular lactate through monocarboxylate transporters (MCTs). This uptake promotes HMGB1 lactylation via a mechanism dependent on p300/CREB-binding protein (CBP). Additionally, lactate inhibits the activity of the deacetylase SIRT1, facilitating the recruitment of p300/CBP to the nucleus, which in turn promotes HMGB1 acetylation. The lactylated and acetylated HMGB1 in macrophages is subsequently released via exosomes, leading to disruption of endothelial integrity and increased vascular permeability. This cascade results in endothelial barrier dysfunction, further advancing the progression of sepsis.
This research elucidates that upon the uptake of exogenous lactate, macrophages activate multiple pathways, resulting in various acyl modifications at distinct lysine residues of HMGB1. These modifications collectively participate in the regulatory mechanisms of cellular functions.
Conclusion
The findings highlight the intricate role of lactate in mediating post-translational modifications of HMGB1 within macrophages during polymicrobial sepsis. The mechanisms identified provide insights into potential therapeutic targets for mitigating the detrimental effects of sepsis through the modulation of lactate-associated signaling pathways.
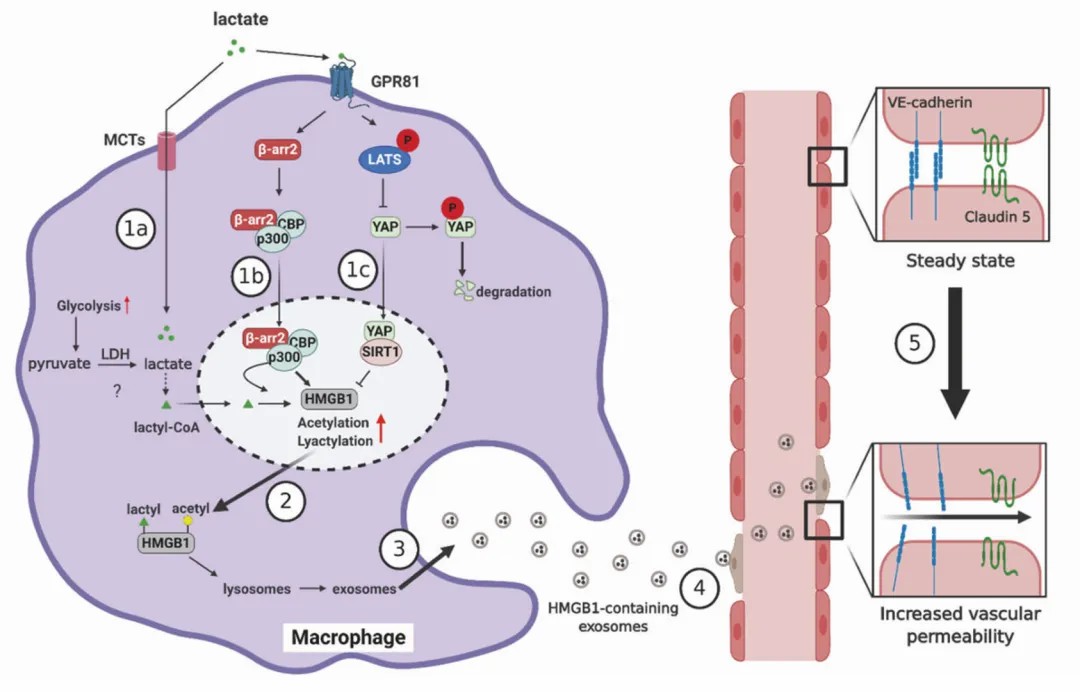 Figure 1 Lactylation and Acetylation Modifications of HMGB1 in Macrophages Increase Vascular Permeability and Exacerbate Polymicrobial Sepsis
Figure 1 Lactylation and Acetylation Modifications of HMGB1 in Macrophages Increase Vascular Permeability and Exacerbate Polymicrobial Sepsis
Case Study 2: Hexokinase 2-Mediated Gene Expression via Histone Lactylation is Required for Hepatic Stellate Cell Activation and Liver Fibrosis
Journal: Cell Metabolism
Publication Date: July 2023
Background
Liver fibrosis represents a wound healing response of the liver to various forms of injury, in which the activation of hepatic stellate cells (HSCs) plays a pivotal role. Recent studies have indicated that metabolic reprogramming through glycolysis is a hallmark of HSC activation, and the inhibition of glycolysis effectively suppresses HSC activation. Lactate produced during HSC activation is implicated in subsequent activation processes; however, the underlying mechanisms linking lactate to HSC activation remain unclear.
Main Findings
The expression of hexokinase 2 (HK2) was found to enhance glycolytic activity and lactate production, influencing gene expression through modifications in histone H3 at lysine 18 (H3K18). Notably, the levels of H3K18 acetylation were comparable between quiescent and in vivo activated HSCs but were suppressed during in vitro HSC activation. Following the knockout of HK2, both lactylation and acetylation modifications of H3K18 were reduced in primary HSCs. Supplementation with exogenous lactate restored lactylation modifications, while further decreasing acetylation. Furthermore, class I histone deacetylase (HDAC) inhibitors facilitated H3K18 acetylation while inhibiting its lactylation, thereby suppressing HSC activation.
These findings provide evidence for a competitive relationship between H3K18 lactylation and acetylation, demonstrating that lactate promotes the expression of genes associated with HSC activation through H3K18 lactylation rather than acetylation, ultimately leading to enhanced HSC activation.
Conclusion
This study elucidates the intricate role of lactate in mediating histone lactylation, which competitively inhibits acetylation in HSCs. The results underscore the potential of targeting lactate-associated pathways to mitigate liver fibrosis by disrupting the activation of hepatic stellate cells.
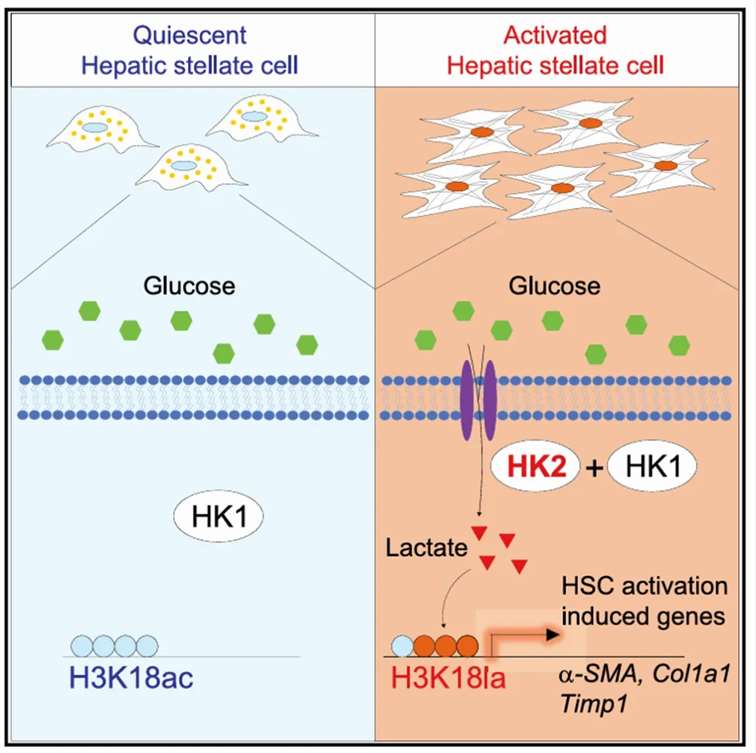 Figure 2 In activated hepatic stellate cells (HSCs), the increased expression of hexokinase 2 (HK2) promotes lactate production, thereby facilitating the lactylation modification of histone H3 at lysine 18 (H3K18) rather than its acetylation. This modification subsequently enhances the expression of genes associated with HSC activation, further driving the activation process of HSCs.
Figure 2 In activated hepatic stellate cells (HSCs), the increased expression of hexokinase 2 (HK2) promotes lactate production, thereby facilitating the lactylation modification of histone H3 at lysine 18 (H3K18) rather than its acetylation. This modification subsequently enhances the expression of genes associated with HSC activation, further driving the activation process of HSCs.
Summary
Lactate levels are regulated by glycolysis and are associated with metabolic reprogramming, gene expression, histone lactylation, and non-histone lactylation, serving as epigenetic modification markers for the glycolytic switch. However, with ongoing research and the expanding diversity of lysine acylation types, increasing evidence has revealed similarities in lysine acylation across substrate proteins, catalytic enzymes, physiological functions, and associated diseases. Moreover, substantial evidence suggests that the activation of complex multiple biological effects likely results in competition among these lysine acylations, significantly augmenting the potential complexity of combinatorial modifications. The crosstalk between different types of acyl modifications remains incompletely understood, and further exploration is required to elucidate the functional interrelationships between acylation and other PTMs.
References
- Lactate and Lactylation: Clinical Applications of Routine Carbon Source and Novel Modification in Human Diseases. Mol Cell Proteomics, 2023 Oct.
- Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ, 2021 Aug.
- Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab, 2023 Jul.
Our products and services are for research use only.