Lysine lactylation (Kla), a post-translational modification (PTM) that emerged in 2019, specifically targets lysine (K) residues. Its initial identification occurred on histones, where it has been scientifically validated as a crucial factor in regulating the homeostasis of M1 macrophages during bacterial infection. As research has progressed, the phenomenon of lactylation on non-histone proteins has also become prominent, thereby broadening the understanding of this novel modification mechanism. Presently, studies on lactylation have become a burgeoning area within the field of protein modifications, guiding a new wave of academic inquiry.
As a dynamic and reversible PTM, lysine lactylation is catalyzed by lactyltransferases, while the removal of lactate is mediated by de-lactylases. Current research has identified several lactyltransferases, including histone acetyltransferase (HAT) p300 and lysine acetyltransferase 8 (KAT8). In contrast, the de-lactylases include histone deacetylases (HDACs) 1-3 and sirtuins (SIRT) 1-3. Notably, in Escherichia coli, the corresponding catalytic enzyme for this process is identified as YiaC, while the de-lactylase is known as CobB. It is imperative to recognize that many additional modifying and demodifying enzymes remain to be explored by the scientific community.
The review article "Lysine Lactylation in the Regulation of Tumor Biology" elucidates the "dual engine" regulatory mechanism of lactylation within biological systems. This mechanism can be divided into two principal components:
Histone Lactylation: This process directly affects histones, altering chromatin structure, regulating the binding of transcription factors, and modulating the accessibility of gene promoter regions, thereby exerting profound effects on gene expression.
Non-Histone Lactylation: The modification of non-histone proteins demonstrates a more complex functional repertoire. It fine-tunes protein functionality through mechanisms such as steric hindrance, conformational changes, and charge neutralization. These regulatory effects encompass various dimensions, including molecular interactions, modulation of enzymatic activity, and subcellular localization, collectively contributing to the extensive impact of lactylation on protein function.
The exploration of lysine lactylation presents significant implications for understanding cellular processes and potential therapeutic avenues in oncology. Further research is warranted to elucidate the comprehensive landscape of lactylation and its myriad roles in health and disease.
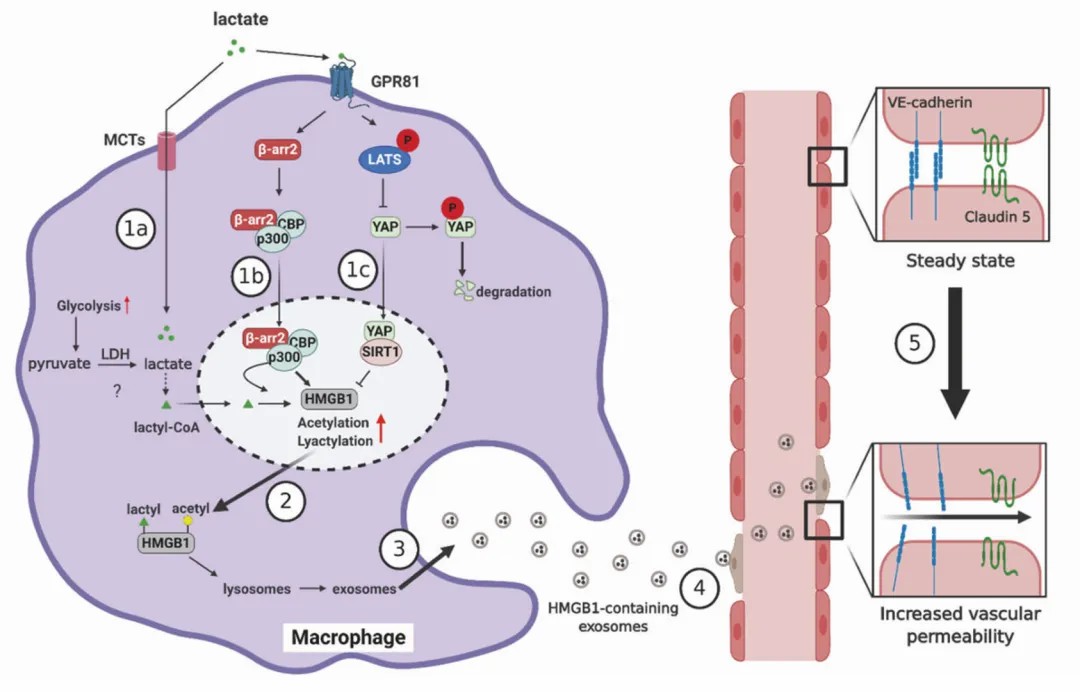
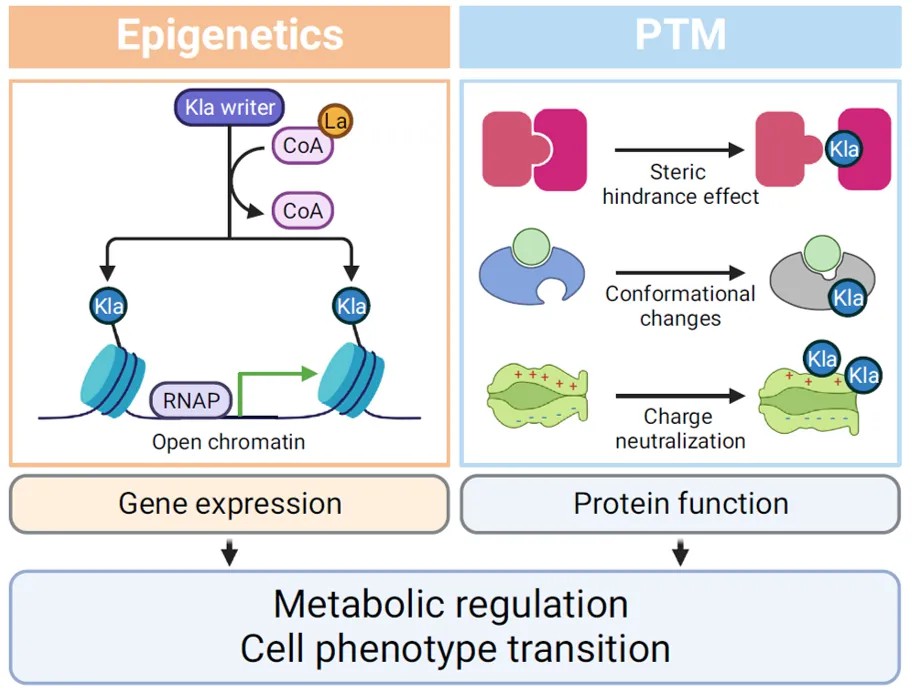 Figure 1: Mechanistic overview of role of lactylation.
Figure 1: Mechanistic overview of role of lactylation.
Advances in Proteomics at Creative Proteomics
Lysine lactylation is an emerging area of study within the field of proteomics, representing a critical PTM that has garnered significant attention. As a high-throughput methodology, modified proteomics has long been the preferred technique for elucidating modification characteristics and changes. To expedite research in lactylation, Creative Proteomics has introduced a groundbreaking Data-Independent Acquisition (DIA) service tailored specifically for lactylated proteins. This service integrates highly specific lactylation antibody-conjugated resin for the enrichment of lactylated peptides, complemented by advanced high-resolution mass spectrometry (MS) detection, facilitating unprecedented depth in the identification of lactylation sites.
Advantage One: Enhanced Detection Capacity through High-Resolution Mass Spectrometry
The introduction of high-resolution mass spectrometry has resulted in a significant increase in the detection of lactylation sites, surpassing the conventional limitations of traditional platforms. In a diverse array of sample types, the number of detectable lactylation sites has exceeded 10,000, with certain projects identifying more than 10,000 distinct sites. This achievement demonstrates an unprecedented capability in lactylation detection.
Advantage Two: Comprehensive Bioinformatics Analysis to Accelerate Mechanistic Insights
Beyond fundamental analyses—such as modification site statistics, differential modification analysis, motif analysis, subcellular localization, domain/Gene Ontology (GO) functional enrichment, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment, and protein-protein interaction (PPI) analysis—this service offers extensive examination of critical regulatory proteins, including transcription factors and kinases. Furthermore, the incorporation of weighted gene co-expression network analysis (WGCNA) provides comprehensive solutions to the pressing need for in-depth data mining, thereby facilitating a deeper understanding of lactylation mechanisms.
By leveraging these innovative approaches, researchers are positioned to explore the complexities of lysine lactylation with enhanced accuracy and detail, contributing to a more nuanced understanding of its biological implications.
Feature Analysis
1. In-Depth Analysis of Transcription Factors: A Novel Perspective on Gene Expression Regulation
Transcription factors, as a distinct class of proteins, possess the capability to bind DNA with sequence specificity, thereby playing a crucial role in transcriptional regulation. These factors not only serve as core components of gene expression regulatory networks but also directly influence cellular fate and the adaptive responses of cells to external environments, underscoring their paramount significance.
Recent research in the context of myocardial infarction has uncovered a notable discovery regarding the specific mechanism of the TGF-β transcription factor family member, Snail1. Evidence suggests that the lactylation of Snail1 significantly enhances its nuclear translocation, subsequently activating downstream signaling pathways. This finding provides new insights into the understanding of disease mechanisms. Given the unique position and widespread influence of transcription factors in gene regulation, this analysis aims to delve into the characteristics and implications of these critical proteins.
2. Kinase Analysis: Delving into Regulatory Mechanisms of Signaling Pathways
Kinases, as a critical class of enzymes within biological systems, are primarily responsible for the transfer of phosphate groups from high-energy donor molecules, such as adenosine triphosphate (ATP), to specific substrates. This catalytic process facilitates the phosphorylation of substrate proteins, enabling precise regulation of their activity. Such phosphorylation not only activates or inhibits the functional capacity of these proteins but also serves as a pivotal molecular switch in the activation and inhibition of cellular signaling pathways, thus attracting significant attention from the scientific community.
It is noteworthy that kinases, as members of the protein family, exhibit activity that is intricately regulated by lactylation modifications. Current research has elucidated that lactylation on kinases can directly influence their active states. For instance, an increase in lactylation at the K62 site of pyruvate kinase M2 (PKM2) significantly enhances its enzymatic activity, thereby promoting the transition of macrophages toward a reparative phenotype. This discovery not only deepens the understanding of kinase regulatory mechanisms but also provides novel insights and potential targets for the treatment and intervention of related diseases.
Given the critical role of lactylation in regulating kinase activity, the present study aims to conduct an in-depth analysis of lactylated kinases. The objective is to further elucidate their specific roles in signaling pathway regulation and to assess their potential application value in therapeutic contexts.
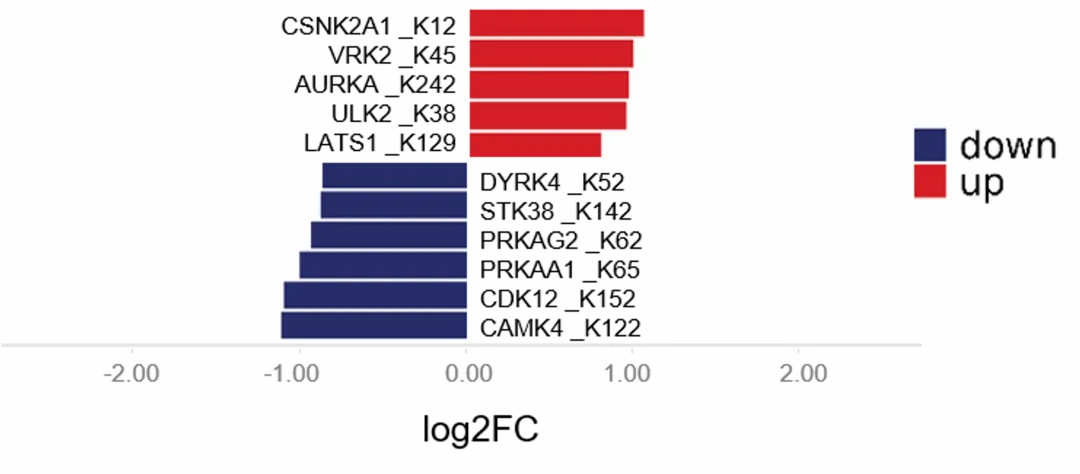 Figure 2: Upregulation and downregulation of lactylation kinases illustrated via a butterfly diagram.
Figure 2: Upregulation and downregulation of lactylation kinases illustrated via a butterfly diagram.
3. Phenotype and Omics Correlation Analysis
WGCNA was employed to systematically explore gene modules characterized by high co-expression. This approach facilitates a detailed investigation into the potential associations between these gene networks and specific phenotypes, as well as the identification of core genes that play pivotal roles within the networks. This methodology is particularly effective for handling complex datasets derived from multiple groups and large cohorts.
Application of the Lactylated Proteome
1. In-depth Analysis of Protein Lactylation Characteristics in Physiological and Pathological Environments
As an emerging form of post-translational modification, lactylation is not only prevalent among histones but also widely observed in non-histone proteins. Numerous studies have elucidated the pivotal role of lactylation in physiological processes and its involvement in various diseases, including tumors, cardiovascular diseases, inflammation, and immune responses. Therefore, a primary research focus is to thoroughly delineate the distinctive characteristics of the lactylated proteome under both physiological and pathological conditions. Furthermore, integrating the study of the lactylated proteome with proteomics holds promise for revealing the complex relationships between lactylation modifications and protein expression, whether they exhibit synergistic or independent variations.
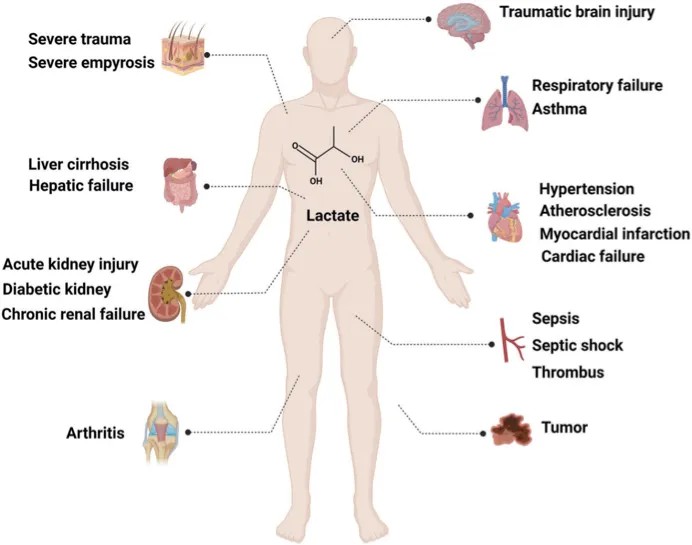 Figure 3: The involvement of lactate and lactylation in various diseases
Figure 3: The involvement of lactate and lactylation in various diseases
2. In-depth Exploration of the Molecular Mechanisms of Lactylation in Physiological and Pathological Contexts
The specific roles of lactylation in physiological and pathological processes, as well as the mechanisms by which lactylation operates through specific proteins, represent critical areas for further investigation. By utilizing lactylated proteome technology to accurately identify differential lactylation sites, researchers can trace back to the sources of the modifying or demodifying enzymes. Additionally, downstream molecules and pathways regulated by lactylation can be explored. This comprehensive investigation into the molecular mechanisms associated with lactylation is emerging as a prominent trend within the current scientific research landscape.
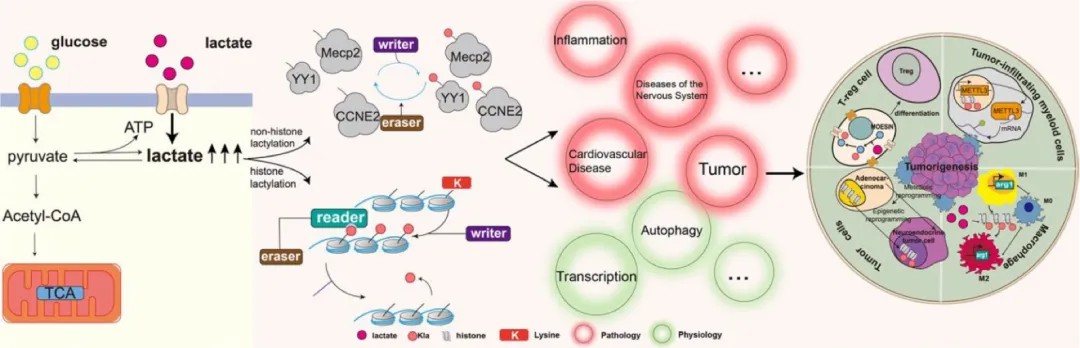 Figure 4: Mechanistic pathways of lactate production and the role of lactylation in disease.
Figure 4: Mechanistic pathways of lactate production and the role of lactylation in disease.
3. Investigation of Crosstalk and Competitive Modifications between Lactylation and Other Acylation Modifications
Lactylation, along with various forms of acylation—including acetylation, succinylation, and butyrylation—occurs specifically on lysine (K) residues. These modification processes share certain enzymes responsible for modification and demodification, revealing a degree of commonality. Moreover, both lactylation and acetylation can regulate gene expression through histones, while also influencing the functionality of non-histone proteins. Consequently, the phenomenon of crosstalk, or competitive modification, among these modifications represents a field of significant research interest.
4. Research on Disease Protein Genomics
Traditional protein genomics research encompasses both proteomics and phosphoproteomics, which may integrate other omics data based on specific research needs or relevance. Given the increasing recognition of the biological significance of protein lactylation, it is anticipated that the lactylated proteome will emerge as a vital component within the domain of disease protein genomics. This is particularly relevant in oncology, where the unique aerobic glycolysis metabolic pattern characteristic of tumor cells, known as the Warburg effect, facilitates the accumulation of lactate within the tumor microenvironment. This lactate subsequently participates in the molecular regulatory networks of tumors via lactylation mechanisms. Therefore, it is reasonable to assert that lactylated proteome technology will serve as a crucial candidate omics approach, exerting a substantial impact on disease protein genomics research.
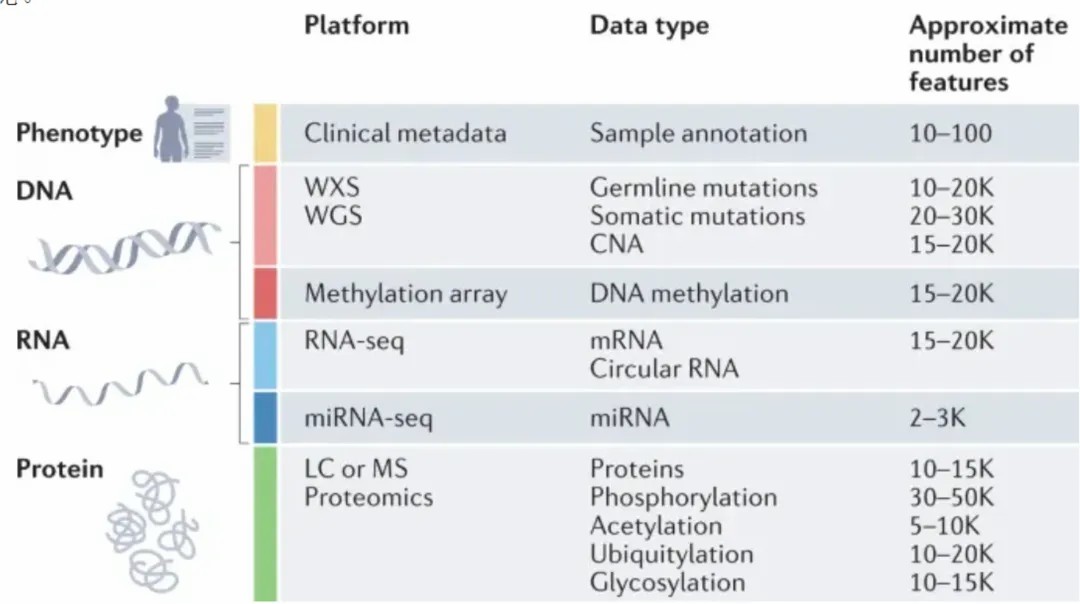 Figure 5: A representation of classical proteomic datasets.
Figure 5: A representation of classical proteomic datasets.
Reference
- Zhang D, Tang Z, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575-580.
- Xie B, Zhang M, Li J, et al. KAT8-catalyzed lactylation promotes eEF1A2-mediated protein synthesis and colorectal carcinogenesis. Proc Natl Acad Sci U S A. 2024;121(8)
- Wang J, Wang Z, Wang Q, Li X, Guo Y. Ubiquitous protein lactylation in health and diseases. Cell Mol Biol Lett. 2024;29(1):23.
- Yang Z, Zheng Y, Gao Q. Lysine lactylation in the regulation of tumor biology. Trends Endocrinol Metab. Published online February 22, 2024.
- Fan M, Yang K, Wang X, et al. Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci Adv. 2023;9(51)
- Wang J, Yang P, Yu T, et al. Lactylation of PKM2 suppresses inflammatory metabolic adaptation in pro-inflammatory macrophages. Int J Biol Sci. 2022;18(16):6210-6225.
- Li X, Yang Y, Zhang B, et al. Lactate metabolism in human health and disease. Signal Transduct Target Ther. 2022;7(1):305. Signal Transduct Target Ther. 2022 Oct 31;7(1):372. doi: 10.1038/s41392-022-01206-5.
- Shang S, Liu J, Hua F. Protein acylation: mechanisms, biological functions and therapeutic targets. Signal Transduct Target Ther. 2022;7(1):396.
- Mani DR, Krug K, Zhang B, et al. Cancer proteogenomics: current impact and future prospects. Nat Rev Cancer. 2022;22(5):298-313.
Our products and services are for research use only.