
- Home
- PTMs Proteomics
- Modificated PROTACs Proteomics Service
- MRM Targeted Quantitative Proteomics For Evaluating PROTAC Efficacy
MRM (Multiple Reaction Monitoring) protein absolute quantification technology can be used to validate non-targeted proteomics results, perform absolute quantification of multiple proteins/peptides, and study pharmacokinetics to reveal oral bioavailability of drugs, drug stability, etc.
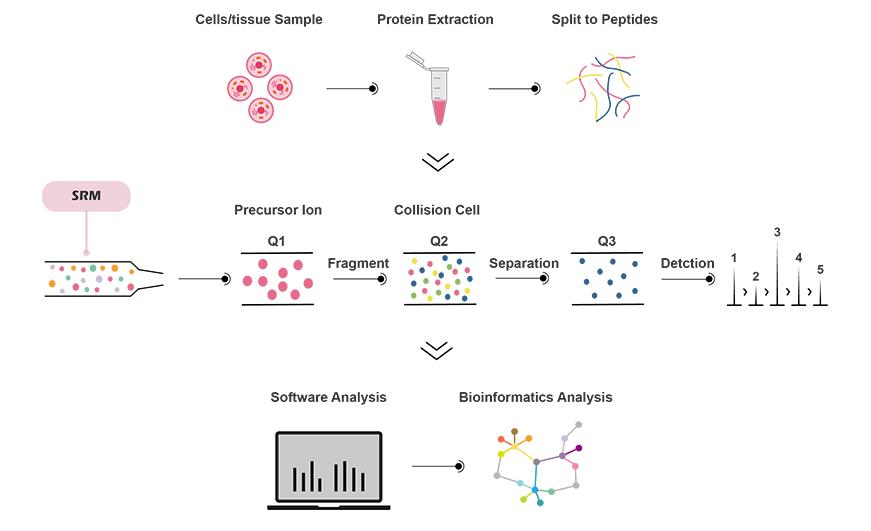
MRM is a quantitative method for targeted proteomics analysis. Based on the sequence information of target peptides, MRM selectively analyzes proteins associated with the target peptide signals, eliminating interference from other peptides. MRM has the highest accuracy among all proteomics quantification methods, allowing for absolute quantification of proteins using reference internal standards and simultaneous analysis of multiple proteins.
| Sample | Amount |
|---|---|
| Cell | 1*107 cells |
| Animal tissue | 1g |
| Plant tissue | 200mg |
| Blood | EDTA added, 1ml |
| Serum | 0.2-0.5ml |
| Urine | 2ml |
| Microbes | Dry weighed 200mg |
| The amount of protein of sample should be no less than 100μg. | |
Provides the highest specificity, sensitivity, and reproducibility for proteomic quantification.
Wide dynamic range, covering protein abundances across 4 orders of magnitude.
High throughput, capable of simultaneously identifying up to 200 specific proteins.
Allows absolute quantification of multiple proteins without the need for antibodies.
Simple, minimizes sample loss, and is amenable to automation.
Exhibits good linearity with acceptable accuracy, precision, matrix effects, process efficiency, and recovery.
Validate non-targeted proteomic results, such as Label-Free proteomics.
Enable absolute quantification of multiple proteins/peptides, analyzing highly homologous members of protein families.
Analyze post-translational modifications of proteins.
Discover and quantitatively analyze biomarkers.
Quantify drugs.
Use in pharmacokinetic studies.
Reveal the oral bioavailability of drugs; drug stability, etc.
Quantification of ARV-110 in rat and mouse plasma for measurement of blood drug concentration, applied in pharmacokinetic studies to improve the oral bioavailability of ARV-110.
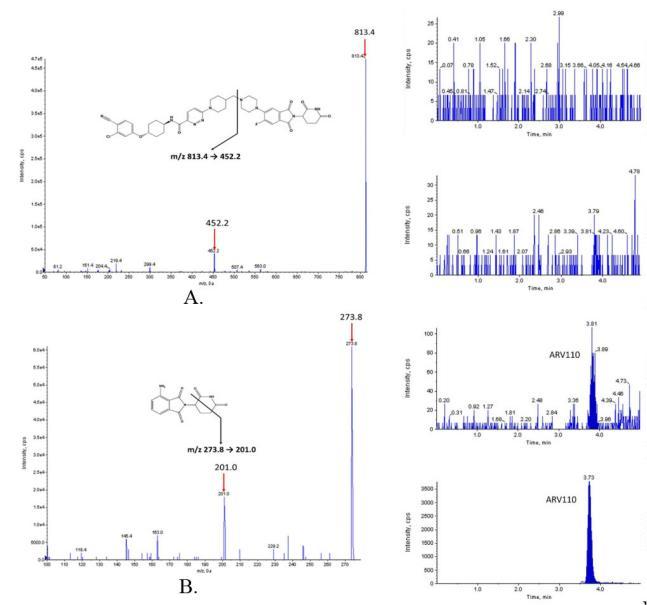
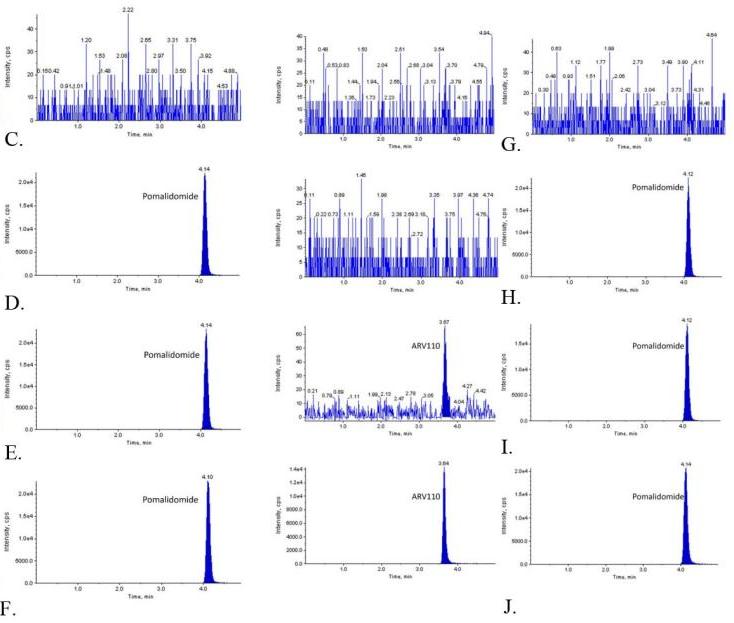
MRM quantification of ARV-110 in rat and mouse plasma and its application in pharmacokinetic studies to improve the oral bioavailability of ARV-110.
A. Representative ion spectra of ARV-110; B. Positive ion mode spectrum of pomalidomide; C. MRM LC-MS/MS chromatograms of ARV-110 (left) and pomalidomide (right) in blank rat plasma after protein precipitation; D. Zero calibrator; E. Lower limit of quantification (LLOQ); F. Rat plasma samples obtained 60 minutes after administration of ARV-110 at a dose of 2 mg/kg; G. MRM LC-MS/MS chromatograms of ARV-110 (left) and pomalidomide (right) in blank mouse plasma after protein precipitation; H. Zero calibrator; H. LLOQ; Mouse plasma samples obtained 60 minutes after administration of ARV-110 at a dose of 2 mg/kg.
Extensive non-targeted protein detection using DIA and TMT.
Targeted quantitative detection using MRM/PRM.
AP-MS for protein-protein interaction analysis.
Ubiquitin proteomics for studying ubiquitination-mediated protein degradation.
Protein quantification using Simple/Digital Western technology.
Our products and services are for research use only.